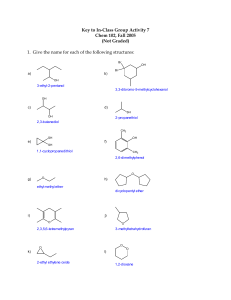
CHEMISTRY 122 HW CH#5 ALCOHOLS 5-10
advertisement
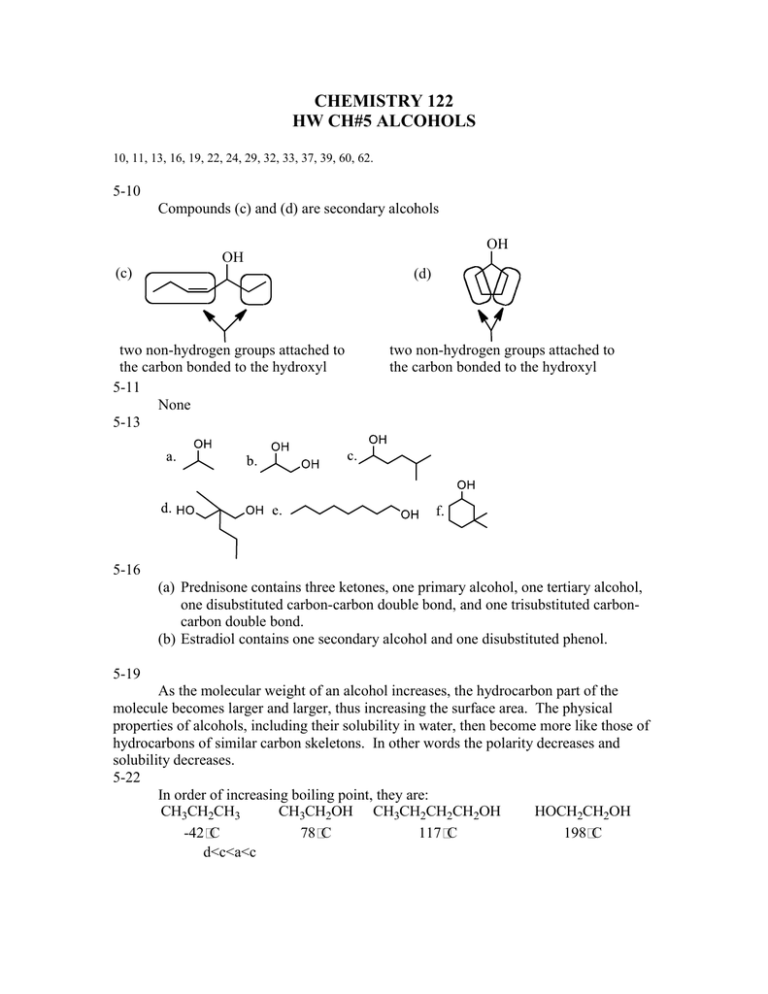
CHEMISTRY 122 HW CH#5 ALCOHOLS 10, 11, 13, 16, 19, 22, 24, 29, 32, 33, 37, 39, 60, 62. 5-10 Compounds (c) and (d) are secondary alcohols OH OH (c) (d) two non-hydrogen groups attached to the carbon bonded to the hydroxyl 5-11 None 5-13 two non-hydrogen groups attached to the carbon bonded to the hydroxyl 5-16 (a) Prednisone contains three ketones, one primary alcohol, one tertiary alcohol, one disubstituted carbon-carbon double bond, and one trisubstituted carboncarbon double bond. (b) Estradiol contains one secondary alcohol and one disubstituted phenol. 5-19 As the molecular weight of an alcohol increases, the hydrocarbon part of the molecule becomes larger and larger, thus increasing the surface area. The physical properties of alcohols, including their solubility in water, then become more like those of hydrocarbons of similar carbon skeletons. In other words the polarity decreases and solubility decreases. 5-22 In order of increasing boiling point, they are: CH3CH2CH3 CH3CH2OH CH3CH2CH2CH2OH HOCH2CH2OH -42 C 78 C 117 C 198 C d<c<a<c 5-24 The cooling effect comes from evaporation. 2-Hexanol's boiling point is too high to be easily evaporated from the skin. 5-29 a. T b. T c. T d. F e.T f.F g. T h. F i. T j. F 5-37 a. H2SO4 f. H2/Pt b. HOH/ H2SO4 g. K2Cr2O7/ H2SO4 c, K2Cr2O7/ H2SO4 d. HBr e. Br2 h. PCC i. K2Cr2O7/ H2SO4 5-39 Ethyl alcohol, found in alcoholic beverages and used as a solvent Ethylene glycol, found in antifreeze 5-40 Isopropyl alcohol, used medicinally as a disinfectant Isopropylene glycol, used as a solvent 5-60 The electronegativity difference between N and H is less than the electronegativity difference between O and H; therefore, the O-H bond is more polar than a N-H bond. Using this information, a N-H----N hydrogen bond is weaker than an O-H----O hydrogen bond. 5-62 The five ether constitutional isomers with molecular formula C5H12O and their common names are: O O Butyl methyl ether O tert-Butyl methyl ether O Isobutyl methyl ether O Ethyl propyl ether sec-Butyl methyl ether O Ethyl isopropyl ether