FURTHER MASS SPECTROMETRY 2015 A guide for A level students
advertisement
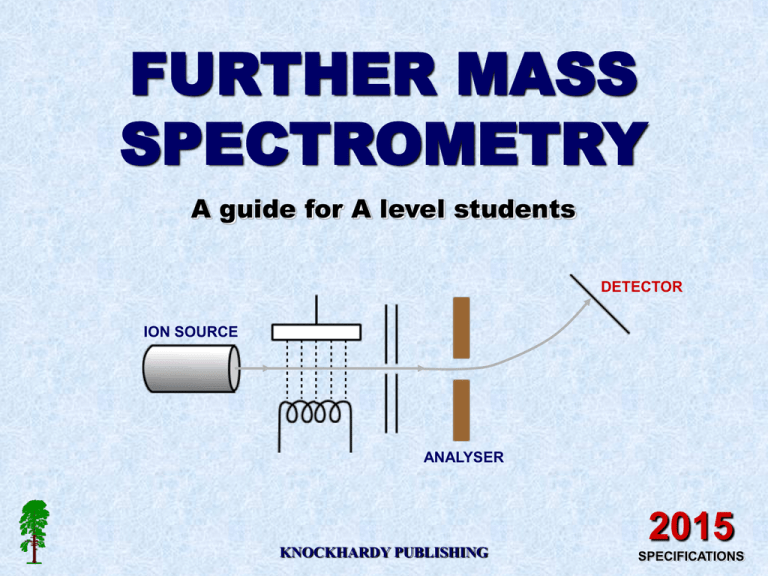
FURTHER MASS SPECTROMETRY A guide for A level students DETECTOR ION SOURCE ANALYSER KNOCKHARDY PUBLISHING 2015 SPECIFICATIONS KNOCKHARDY PUBLISHING MASS SPECTROMETRY INTRODUCTION This Powerpoint show is one of several produced to help students understand selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board is available. Accompanying notes on this, and the full range of AS and A2 topics, are available from the KNOCKHARDY SCIENCE WEBSITE at... www.argonet.co.uk/users/hoptonj/sci.htm Navigation is achieved by... either clicking on the grey arrows at the foot of each page or using the left and right arrow keys on the keyboard MASS SPECTROMETRY CONTENTS • Fragmentation of molecular ions - theory • What a mass spectrum tells you • Molecular ions • Fragmentation • Mass spectra of alkanes • Mass spectra of halogenoalkanes • Mass spectra of aldehydes and ketones • Test questions • Check list MASS SPECTROMETRY Before you start it would be helpful to… • recall the basic principles of a mass spectrometer • know the different types of functional group in organic chemsitry MOLECULAR MASS DETERMINATION USING MASS SPECTROMETRY Nowadays, mass spectrometry is used to identify unknown or new compounds. IONISATION When a molecule is ionised it forms a MOLECULAR ION which can also undergo FRAGMENTATION or REARRANGEMENT to produce particles of smaller mass. MOLECULAR ION FRAGMENTION Only particles with a positive charge will be deflected and detected. RE-ARRANGEMENT FRAGMENTION The resulting spectrum has many peaks. The final peak (M+) shows the molecular ion (highest m/z value) and indicates the molecular mass. The rest of the spectrum provides information about the structure. THE MASS SPECTRUM Spectra obtained for organic molecules have many peaks. Each peak is due to a particular fragment with a certain m/z value. highest m/z value usually corresponds to the molecular ion its position provides information about the molecular mass of a substance the tallest peaks come from the most stable species THE MASS SPECTRUM Spectra obtained for organic molecules have many peaks. Each peak is due to a particular fragment with a certain m/z value. highest m/z value usually corresponds to the molecular ion its position provides information about the molecular mass of a substance the tallest peaks come from the most stable species Interpretation of thousands of spectra has shown that many classes of organic compound show characteristic fragmentation patterns due to their functional groups. It is possible to identify the type of compound from its spectrum by looking at the ... position of peaks differences between major peaks THE MASS SPECTRUM - THE MOLECULAR ION In the spectrum of octane, a signal occurs at 114 due to the species C8H18+ 80 60 40 molecular ion 114 20 Abundance % 100 The species due to the final signal is known as the molecular ion and is usually corresponds to the molecular mass of the compound. 0 . 10 20 30 40 50 60 70 80 90 100 110 120 m/z 130 140 THE MASS SPECTRUM - THE MOLECULAR ION 80 60 40 114 20 Abundance % 100 The small peak (M+1) at 115 due to the natural abundance (about 1%) of carbon-13. The height of this peak relative to that for the molecular ion depends on the number of carbon atoms in the molecule. The more carbons present, the larger the M+1 peak. 0 . 10 20 30 40 50 60 70 80 90 100 110 120 m/z 130 140 THE MASS SPECTRUM - FRAGMENTATION 60 80 43 29 71 85 40 57 114 20 Abundance % 100 The rest of the spectrum provides additional information of the molecule’s structure. Peaks appear due to characteristic fragments (e.g. 29 due to C2H5+) and differences between two peaks also indicates the loss of certain units (18 for H2O, 28 for CO). 0 . 10 20 30 40 50 60 70 80 90 100 110 120 m/z 130 140 FRAGMENTATION PATTERNS ALKANES The mass spectra of simple hydrocarbons have peaks at m/z values corresponding to the ions produced by breaking C-C bonds. Peaks can occur at ... m/z 15 CH3+ 29 C2H5+ 43 C3H7+ 57 C4H9+ 71 C5H11+ 85 C6H13+ etc. • the stability of the carbocation formed affects its abundance • the more stable the cation the higher the peak • the more alkyl groups attached to the carbocation the more stable it is most stable tertiary 3° > secondary 2° > primary 1° least stable alkyl groups are electron releasing and stabilise the cation FRAGMENTATION PATTERNS HALOGENOALKANES 81Br 40 60 80 molecular ion contains...79Br 20 Abundance % 100 Multiple peaks occur in the molecular ion region due to different halogen isotopes. There are two peaks for the molecular ion of C2H5Br, one for the molecule containing the isotope 79Br and the other for the one with the 81Br isotope. Because the two isotopes are of similar abundance, the peaks are of similar height. 0 m/z 10 20 30 40 50 60 70 80 90 100 110 120 130 140 FRAGMENTATION PATTERNS ALDEHYDES AND KETONES Cleavage of bonds next to the carbonyl group (C=O) is a characteristic fragmentation of aldehydes and ketones. A common fragment is carbon monoxide (CO) but as it is a molecule and thus uncharged it will not produce a peak of its own. However, it will produce an m/z drop of 28 somewhere in the spectrum. The position of the carbonyl group influences the fragmentation pattern because the molecular ion fragments either side of the carbonyl group the more stable the acylium ion RCO+, the more abundant it will be and the more abundant the species the taller its peak in the mass spectrum FRAGMENTATION PATTERNS Aldehydes and ketones The position of the carbonyl group influences the fragmentation pattern because the molecular ion fragments either side of the carbonyl group. O CH3 C •+ C4H9 MOLECULAR ION has m/z = 100 FRAGMENTATION PATTERNS Aldehydes and ketones The position of the carbonyl group influences the fragmentation pattern because the molecular ion fragments either side of the carbonyl group. O CH3 C •+ C4H9 MOLECULAR ION has m/z = 100 O C4H9 C+ CH3• m/z = 85 O C4H9 C• CH3+ m/z = 15 Breaking the bond between the methyl group and the carbonyl group produces two possible ions, depending on how the bond breaks. Two peaks at m/z values 15 and 85 will appear in the mass spectrum. FRAGMENTATION PATTERNS Aldehydes and ketones The position of the carbonyl group influences the fragmentation pattern because the molecular ion fragments either side of the carbonyl group. O CH3 C •+ C4H9 MOLECULAR ION has m/z = 100 O Breaking the bond between the butyl group and the carbonyl group produces two further ions, depending on how the bond breaks. Two peaks at m/z values 43 and 57 will appear in the mass spectrum. CH3 C+ C4H9• m/z = 43 O CH3 C• C4H9+ m/z = 57 FRAGMENTATION PATTERNS Aldehydes and ketones The position of the carbonyl group influences the fragmentation pattern because the molecular ion fragments either side of the carbonyl group. O Example; CH3 C •+ C4H9 O C4H9 C+ MOLECULAR ION has m/z = 100 O CH3• m/z = 28 CH3 C+ m/z = 85 m/z = 43 O O C4H9 C• CH3+ CH3 C• m/z = 15 A further peak occurs at m/z = 72 (100-28) due to loss of CO C4H9• C4H9+ m/z = 57 IDENTIFY THE COMPOUNDS IDENTIFY THE COMPOUND 80 40 60 29 122 20 Abundance % 100 43 124 79 81 0 m/z 10 20 30 40 50 60 70 80 90 100 110 120 130 140 IDENTIFY THE COMPOUND 80 40 60 29 122 20 Abundance % 100 43 124 79 81 0 m/z 10 20 30 40 50 60 70 80 C3H7Br 90 100 110 120 130 140 80 105 77 60 51 120 40 43 28 20 Abundance % 100 IDENTIFY THE COMPOUND 0 m/z 10 20 30 40 50 60 70 80 90 100 110 120 130 140 80 105 77 60 51 120 40 43 28 20 Abundance % 100 IDENTIFY THE COMPOUND 0 m/z 10 20 30 40 50 60 70 80 90 C6H5COCH3 100 110 120 130 140 60 80 105 106 40 51 77 57 43 20 Abundance % 100 IDENTIFY THE COMPOUND 28 0 m/z 10 20 30 40 50 60 70 80 90 100 110 120 130 140 60 80 105 106 40 51 77 57 43 20 Abundance % 100 IDENTIFY THE COMPOUND 28 0 m/z 10 20 30 40 50 60 70 80 90 C6H5CHO 100 110 120 130 140 56 57 113 60 80 43 40 71 142 20 Abundance % 100 IDENTIFY THE COMPOUND 0 m/z 10 20 30 40 50 60 70 80 90 100 110 120 130 140 56 57 113 60 80 43 40 71 142 20 Abundance % 100 IDENTIFY THE COMPOUND 0 m/z 10 20 30 40 50 60 70 80 C10H22 90 100 110 120 130 140 REVISION CHECK What should you be able to do? Understand how mass spectrometry can be used to calculate molecular mass Recall the term molecular ion and understand what information it provides Interpret simple mass spectra CAN YOU DO ALL OF THESE? YES NO You need to go over the relevant topic(s) again Click on the button to return to the menu WELL DONE! Try some past paper questions FURTHER MASS SPECTROMETRY The End © 2015 JONATHAN HOPTON & KNOCKHARDY PUBLISHING