EDTA Titrations Introduction 1.) Metal Chelate Complexes
advertisement
EDTA Titrations
Introduction
1.) Metal Chelate Complexes
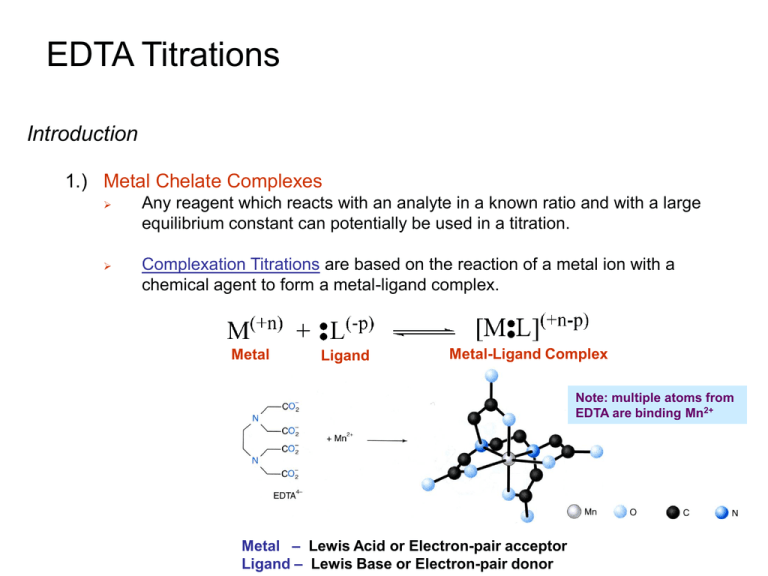
Any reagent which reacts with an analyte in a known ratio and with a large
equilibrium constant can potentially be used in a titration.
Complexation Titrations are based on the reaction of a metal ion with a
chemical agent to form a metal-ligand complex.
Metal
Ligand
Metal-Ligand Complex
Note: multiple atoms from
EDTA are binding Mn2+
Metal – Lewis Acid or Electron-pair acceptor
Ligand – Lewis Base or Electron-pair donor
EDTA Titrations
Introduction
1.) Metal Chelate Complexes
Complexation Titrations are essentially a Lewis acid-base reaction, in which
an electron pair is donated from one chemical to another
The ligands used in complexometric titrations are also known as chelating
agents.
-
Ligand that attaches to a metal ion through more than one ligand atom
Most chelating agents contain N or O
-
Elements that contain free electron pairs that may be donated to a metal
Fe-DTPA Complex
EDTA Titrations
Metal Chelation in Nature
1.) Potassium Ion Channels in Cell Membranes
Electrical signals are essential for life
Electrical signals are highly controlled by the selective passage of ions across
cellular membranes
-
Ion channels control this function
Potassium ion channels are the largest and most diverse group
Used in brain, heart and nervous system
channel contains
pore that only
allows K+ to pass
K+ is chelated by O
in channel
K+ channel spans membrane
Opening of potassium channel allows K+ to exit cell
and change the electrical potential across membrane
Current Opinion in Structural Biology 2001, 11:408–414
http://www.bimcore.emory.edu/home/molmod/Wthiel/Kchannel.html
EDTA Titrations
Metal –Chelate Complexes
1.) Formation Constant (Kf)
The equilibrium constant for the reaction between a metal ion (M+n) and a
chelating agent (L-P) is known as a formation constant or stability constant.
Applying different and specific names to the general equilibrium constant is a
common occurrence
-
Solubility (Ksp), acid-base (Ka, Kb), water dissociation (Kw), etc
Chelate effect: ability of multidentate ligands to form stronger metal
complexes compared to monodentate ligands.
Kf = 8x109
Kf = 4x109
2 ethylenediamine molecules binds tighter than 4 methylamine molecules
EDTA Titrations
Metal –Chelate Complexes
2.) Chelate Effect
Usually chelating agents with more than one electron pair to donate will form
stronger complexes with metal ions than chelating agents with only one
electron pair.
-
Multidentate ligand: a chelating agent with more than one free electron pair
-
Typically more than one O or N
Larger Kf values
Stoichiometry is 1:1 regardless of the ion charge
Monodentate ligand: a chelating agent with only one pair of free electrons
Multidentate ligand that binds radioactive metal attached
to monoclonal antibody (mAb).
mAb is a protein that binds to a specific feature on a
tumor cell delivering toxic dose of radiation.
EDTA Titrations
EDTA
1.) EDTA (Ethylenediaminetetraacetic acid)
One of the most common chelating agents used for complexometric titrations
in analytical chemistry.
EDTA has 6 nitrogens & oxygens in its structure giving it 6 free electron pairs
that it can donate to metal ions.
-
High Kf values
6 acid-base sites in its structure
EDTA Titrations
EDTA
2.) Acid-Base Forms
EDTA exists in up to 7 different acid-base forms depending on the solution
pH.
The most basic form (Y4-) is the one which primarily reacts with metal ions.
EDTA-Mn Complex
EDTA Titrations
EDTA
2.) Acid-Base Forms
aY 4
6
Fraction (a) of the most basic form of EDTA (Y4-) is defined by the H+
concentration and acid-base equilibrium constants
5
4
3
K1K 2 K 3 K 4 K5 K6
{[H ] [H ] K1 [H ] K1K 2 [H ] K1K 2 K 3 [H ]2 K1K 2 K 3 K 4 [H ]K1K 2 K 3 K 4 K5 K1K 2 K 3 K 4 K 5 K6 }
Fraction (a) of EDTA in the form Y4-:
aY 4
aY 4
[Y 4 ]
[H6Y 2 ] [H5Y ] [H 4Y ] [H 3Y ] [H2Y 2 ] [HY 3 ] [Y 4 ]
[Y 4 ]
EDTA
where [EDTA] is the total concentration of all free EDTA species in solution
aY4- is depended on the pH of the solution
EDTA Titrations
EDTA
3.) EDTA Complexes
The basic form of EDTA (Y4-) reacts with most metal ions to form a 1:1
complex.
-
Other forms of EDTA will also chelate metal ions
Kf
[MY n- 4 ]
[M n ][Y 4 ]
Note: This reaction only involves Y4-, but not the other forms of EDTA
Recall: the concentration of Y4- and the total concentration of EDTA is
solution [EDTA] are related as follows:
[Y 4 ] aY 4 EDTA
where aY4-is dependent on pH
EDTA Titrations
EDTA
3.) EDTA Complexes
The basic form of EDTA (Y4-) reacts with most metal ions to form a 1:1
complex.
EDTA Titrations
EDTA
3.) EDTA Complexes
[Y
4
Substitute [Y4-] into Kf equation
] aY 4 EDTA
Kf
Kf
[MY n- 4 ]
[M n ]a Y 4- [EDTA]
[MY n- 4 ]
[M n ][Y 4 ]
where [EDTA] is the total
concentration of EDTA added
to the solution not bound to
metal ions
If pH is fixed by a buffer, then aY4- is a constant that can be combined with Kf
Conditional or effective formation constant:
(at a given pH)
K'f
K K f a Y 4-
[MY n- 4 ]
[M n ][EDTA]
EDTA Titrations
EDTA
3.) EDTA Complexes
Assumes the uncomplexed EDTA were all in one form
K'f K f a Y 4at any pH, we can find aY4- and evaluate Kf’
EDTA Titrations
EDTA
4.) Example:
What is the concentration of free Fe3+ in a solution of 0.10 M Fe(EDTA)- at pH
8.00?
EDTA Titrations
EDTA
5.) pH Limitation
Note that the metal –EDTA complex becomes less stable as pH decreases
-
Kf decreases
[Fe3+] = 5.4x10-7 at pH 2.0 -> [Fe3+] = 1.4x10-12 at pH 8.0
In order to get a “complete” titration (Kf ≥106), EDTA requires a certain
minimum pH for the titration of each metal ion
End Point becomes less distinct as pH is
lowered, limiting the utility of EDTA as a titrant
EDTA Titrations
Minimum pH for Effective
Titration of Metal Ions
EDTA
5.) pH Limitation
By adjusting the pH of an EDTA
titration:
one type of metal ion (e.g. Fe3+) can
be titrated without interference from
others (e.g. Ca2+)
EDTA Titrations
EDTA Titration Curves
1.) Titration Curve
The titration of a metal ion with EDTA is similar to the titration of a strong acid
(M+) with a weak base (EDTA)
K'f K f a Y 4
The Titration Curve has three distinct regions:
-
Before the equivalence point (excess Mn+)
-
At the equivalence point ([EDTA]=[Mn+]
-
After the equivalence point (excess EDTA)
pM log [M n ]
EDTA Titrations
EDTA Titration Curves
2.) Example
What is the value of [Mn+] and pM for 50.0 ml of a 0.0500 M Mg2+ solution
buffered at pH 10.00 and titrated with 0.0500 m EDTA when (a) 5.0 mL, (b)
50.0 mL and (c) 51.0 mL EDTA is added?
Kf = 108.79 = 6.2x108
aY4- at pH 10.0 = 0.30
mL EDTA at equivalence point:
Ve ( mL )0.0500 M 5.00 mL ( 0.0500 M ) Ve 50.00 mL
mmol of EDTA
mmol of Mg2+
EDTA Titrations
EDTA Titration Curves
2.) Example
(a) Before Equivalence Point ( 5.0 mL of EDTA)
Before the equivalence point, the [Mn+] is equal to the concentration of excess
unreacted Mn+. Dissociation of MYn-4 is negligible.
moles of Mg2+
originally present
[Mg
2
moles of EDTA added
[(0 .0500 M Mg2 )(0 .0500 L) - (0 .0500 M EDTA)(0 .0050 L)]
]
[0.0500 L 0.0050 L]
Original volume
solution
Volume titrant
added
Dilution effect
[Mg 2 ] 0.0409 M pMg 2 log [Mg 2 ] 1.39
EDTA Titrations
EDTA Titration Curves
2.) Example
(b) At Equivalence Point ( 50.0 mL of EDTA)
Virtually all of the metal ion is now in the form MgY2-
Original volume of
Original [Mn+]
Mn+ solution
[MgY 2 ] (0 .0500 M )
(0 .0500 L)
(0.0500 L 0.0500 L)
Original volume
solution
[MgY 2 ] 0.0250 M
Moles Mg+ ≡ moles MgY2-
Volume titrant
added
Dilution effect
EDTA Titrations
EDTA Titration Curves
2.) Example
(b) At Equivalence Point ( 50.0 mL of EDTA)
The concentration of free Mg2+ is then calculated as follows:
Initial Concentration (M)
0
0
0.0250
Final Concentration (M)
x
x
0.0250 - x
K'f K f aY 4
[Mg( EDTA)- 2 ]
[Mg2 ][EDTA]
( 0.0250 x )
8
( 6.2 10 )( 0.30 )
( x )( x )
Solve for x using the quadratic equation:
x [Mg 2 ] [EDTA ] 1.16 10 5 pMg 2 4.94
EDTA Titrations
EDTA Titration Curves
2.) Example
(c) After the Equivalence Point ( 51.0 mL of EDTA)
Virtually all of the metal ion is now in the form MgY2- and there is excess,
unreacted EDTA. A small amount of free Mn+ exists in equilibrium with
MgY4- and EDTA.
Calculate excess [EDTA]:
Volume excess
Original [EDTA] titrant
[EDTA]
Excess moles EDTA
(0 .0500 M )(0 .0010 L)
(0.0500 L 0.0510 L)
Original volume
solution
[EDTA ] 4.95 10 4 M
Volume titrant
added
Dilution effect
EDTA Titrations
EDTA Titration Curves
2.) Example
(c) After the Equivalence Point ( 51.0 mL of EDTA)
Calculate [MgY2-]:
Original volume of
Original [Mn+]
Mn+ solution
[MgY 2 ] (0 .0500 M )
(0 .0500 L)
(0.0500 L 0.0510 L)
Original volume
solution
[MgY 2 ] 0.0248 M
Moles Mg+ ≡ moles MgY2-
Volume titrant
added
Only Difference
Dilution effect
EDTA Titrations
EDTA Titration Curves
2.) Example
(c) After the Equivalence Point ( 51.0 mL of EDTA)
[Mg2+-] is given by the equilibrium expression using [EDTA] and [MgY2-]:
K f K f aY 4
'
8
( 6.2 10 )( 0.30 )
[Mg( EDTA)- 2 ]
[Mg2 ][EDTA]
( 0.0248 M )
( x )( 4.95 10 4 M )
x [Mg 2 ] 2.7 10 7 pMg 2 6.57
EDTA Titrations
EDTA Titration Curves
2.) Example
Final titration curve for 50.0 ml of 0.0500 M Mg2+ with 0.0500 m EDTA at pH
10.00.
-
Also shown is the titration of 50.0 mL of 0.0500 M Zn2+
Note: the equivalence point is sharper for Zn2+
vs. Mg2+. This is due to Zn2+ having a larger
formation constant.
The completeness of these reactions is
dependent on aY4- and correspondingly pH.
pH is an important factor in setting the completeness
and selectivity of an EDTA titration
EDTA Titrations
Auxiliary Complexing Agents
1.) Metal Hydroxide
In general, as pH increases a titration of a metal ion with EDTA will have a
higher Kf.
-
Larger change at the equivalence point.
Exception: If Mn+ reacts with OH- to form an insoluble metal hydroxide
Auxiliary Complexing Agents: a ligand can be added that complexes with Mn+
strong enough to prevent hydroxide formation.
-
Ammonia, tartrate, citrate or triethanolamine
Binds metal weaker than EDTA
Fraction of free metal ion (aM) depends on the
equilibrium constants () or cumulative formation
constants:
Use a new conditional formation constant that
incorporates the fraction of free metal:
aM
1
1 1 [ L ] 2 [ L ] 2 n [ L ] n
K'f' aY 4 a Zn 2 K f
EDTA Titrations
Auxiliary Complexing Agents
2.) Illustration:
Titration of Cu+2 (CuSO4) with EDTA
Addition of Ammonia Buffer results in a dark blue solution
-
Cu(II)-ammonia complex is formed
Addition of EDTA displaces ammonia with corresponding color change
CuSO4
Cu-ammonia Cu-EDTA
EDTA Titrations
Metal Ion Indicators
1.) Determination of EDTA Titration End Point
Four Methods:
1.
2.
3.
4.
Potential
Measurements
Metal Ion Indicator: a compound that changes color when it binds to a metal
ion
-
Metal ion indicator
Mercury electrode
pH electrode
Ion-selective electrode
Similar to pH indicator, which changes color with pH or as the compound
binds H+
For an EDTA titration, the indicator must bind the metal ion less strongly than
EDTA
-
Similar in concept to Auxiliary Complexing Agents
Needs to release metal ion to EDTA
End Point indicated by a color
change from red to blue
(red)
(colorless)
(colorless)
(blue)
EDTA Titrations
Metal Ion Indicators
2.) Illustration
Titration of Mg2+ by EDTA
-
Eriochrome Black T Indicator
Addition of EDTA
Before
Near
Equivalence point
After
EDTA Titrations
Metal Ion Indicators
3.) Common Metal Ion Indicators
Most are pH indicators and can only be used over a given pH range
EDTA Titrations
Metal Ion Indicators
3.) Common Metal Ion Indicators
Useful pH ranges
EDTA Titrations
EDTA Titration Techniques
1.) Almost all elements can be determined by EDTA titration
Needs to be present at sufficient concentrations
Extensive Literature where techniques are listed in:
1)
2)
3)
G. Schwarzenbach and H. Flaschka, “Complexometric Titrations”,
Methuen:London, 1969.
H.A. Flaschka, “EDTA Titrations”, Pergamon Press:New York, 1959
C.N. Reilley, A.J. Bernard, Jr., and R. Puschel, In: L. Meites (ed.) “Handbook of
Analytical Chemistry”, McGraw-Hill:New York, 1963; pp. 3-76 to 3-234.
Some Common Techniques used in these titrations include:
a)
b)
c)
d)
e)
Direct Titrations
Back Titrations
Displacement Titrations
Indirect Titrations
Masking Agents
EDTA Titrations
EDTA Titration Techniques
2.) Direct Titrations
Analyte is buffered to appropriate pH and is titrated directly with EDTA
An auxiliary complexing agent may be required to prevent precipitation of
metal hydroxide.
3.) Back Titrations
A known excess of EDTA is added to analyte
-
Free EDTA left over after all metal ion is bound with EDTA
The remaining excess of EDTA is then titrated with a standard solution of a
second metal ion
Approach necessary if analyte:
-
precipitates in the presence of EDTA
Reacts slowly with EDTA
Blocks the indicator
Second metal ion must not displace analyte from EDTA
K f ( analyte )aY 4 K f (sec ond metal ion )aY 4
EDTA Titrations
EDTA Titration Techniques
4.) Displacement Titration
Used for some analytes that don’t have satisfactory metal ion indicators
Analyte (Mn+) is treated with excess Mg(EDTA)2-, causes release of Mg2+.
Requires:
Kf ( M n )aY 4 Kf ( Mg2 )aY 4
Amount of Mg2+ released is then determined by titration with a standard EDTA
solution
Concentration of released Mg2+ equals [Mn+]
EDTA Titrations
EDTA Titration Techniques
5.) Indirect Titration
Used to determine anions that precipitate with metal ions
Anion is precipitated from solution by addition of excess metal ion
-
ex. SO42- + excess Ba2+
Precipitate is filtered & washed
Precipitate is then reacted with excess EDTA to bring the metal ion back into
solution
The excess EDTA is titrated with Mg2+ solution
[Total EDTA] = [MYn-4] + [Y4-]
complex
Known
determine
free
Titrate
EDTA Titrations
EDTA Titration Techniques
6.) Masking Agents
A reagent added to prevent reaction of some metal ion with EDTA
Al3+ is not available to bind EDTA because of the complex with F-
Requires:
Kf ( AlF 3 ) Kf ( Al ( EDTA ))
6
Demasking: refers to the release of a metal ion from a masking agent





