Supercritical Fluid •
advertisement
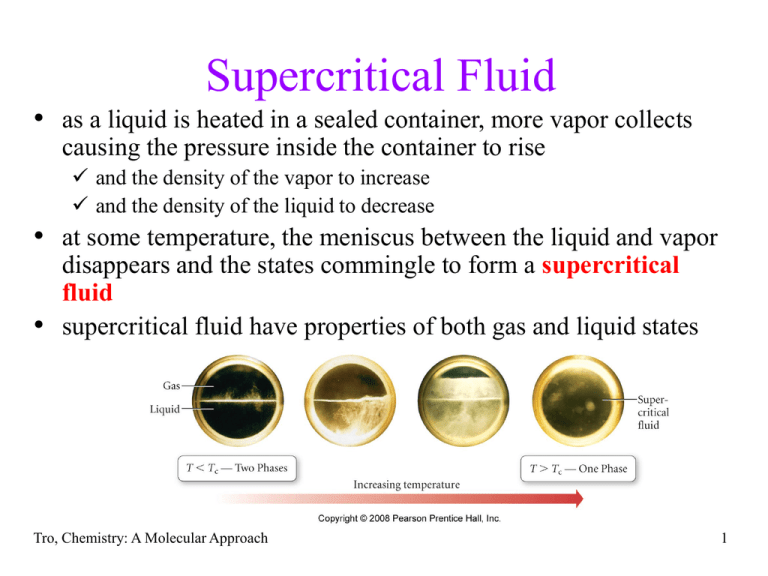
Supercritical Fluid • as a liquid is heated in a sealed container, more vapor collects causing the pressure inside the container to rise and the density of the vapor to increase and the density of the liquid to decrease • at some temperature, the meniscus between the liquid and vapor • disappears and the states commingle to form a supercritical fluid supercritical fluid have properties of both gas and liquid states Tro, Chemistry: A Molecular Approach 1 http://www.youtube.com/watch?v=yBRdBrnIlTQ Tro, Chemistry: A Molecular Approach 2 Tro, Chemistry: A Molecular Approach 3 Tro, Chemistry: A Molecular Approach 4 The Critical Point • the temperature required to produce a supercritical fluid is called the critical temperature • the pressure at the critical temperature is called the critical pressure • at the critical temperature or higher temperatures, the gas cannot be condensed to a liquid, no matter how high the pressure gets Tro, Chemistry: A Molecular Approach 5 Sublimation and Deposition • molecules in the solid have thermal energy that allows • • • them to vibrate surface molecules with sufficient energy may break free from the surface and become a gas – this process is called sublimation the capturing of vapor molecules into a solid is called deposition the solid and vapor phases exist in dynamic equilibrium in a closed container at temperatures below the melting point therefore, molecular solids have a vapor pressure solid Tro, Chemistry: A Molecular Approach sublimation deposition gas 6 Sublimation Tro, Chemistry: A Molecular Approach 7 Tro, Chemistry: A Molecular Approach 8 Melting = Fusion • as a solid is heated, its temperature rises and the molecules vibrate more vigorously • once the temperature reaches the melting point, the molecules have sufficient energy to overcome some of the attractions that hold them in position and the solid melts (or fuses) • the opposite of melting is freezing Tro, Chemistry: A Molecular Approach 9 Heating Curve of Water 10 Phase Diagrams • describe the different states and state changes that occur at various temperature - pressure conditions • areas represent states • lines represent state changes liquid/gas line is vapor pressure curve both states exist simultaneously critical point is the furthest point on the vapor pressure curve • triple point is the temperature/pressure condition where all three states exist simultaneously • for most substances, freezing point increases as pressure increases Tro, Chemistry: A Molecular Approach 11 Phase Diagrams Fusion Curve critical point Pressure melting 1 atm freezing Liquid Solid normal boiling pt. normal melting pt. Sublimation Curve triple point vaporization condensation sublimation Gas deposition Temperature Vapor Pressure Curve 12 Tro, Chemistry: A Molecular Approach 13 Pressure Phase Diagram of Water 1 atm critical point 374.1°C 217.7 atm Ice Water normal melting pt. 0°C normal boiling pt. 100°C triple point 0.01°C 0.006 atm Steam Temperature 14 Water – An Extraordinary Substance • water is a liquid at room temperature most molecular substances with small molar masses are gases at room temperature due to H-bonding between molecules • water is an excellent solvent – dissolving many ionic and polar molecular substances because of its large dipole moment even many small nonpolar molecules have solubility in water e.g., O2, CO2 • water has a very high specific heat for a molecular substance moderating effect on coastal climates • water expands when it freezes at a pressure of 1 atm about 9% making ice less dense than liquid water Tro, Chemistry: A Molecular Approach 15