EOC review III
advertisement

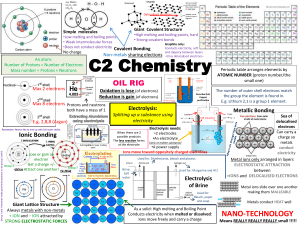
EOC review III What type of bond is in methane? Write lewis structure for the following. F2 N2 Br2 H2 What is the type and geometric shape of the bonds for water. Which contains nonpolar bonds and is a nonpolar molecule? Methane florine hydrofloric acid hydrochloric acid What is the % mass of oxygen in nitric acid? Define empirical formula… Calculate empirical formula for 8.52g of carbon, 1.42g of hydrogen. What is the mass of 4.2 moles of NO2? What does hydrate or hydrant mean? If empirical formula is CH3 what could be the molecular formula? List characteristics of: ionic compound molecular/covalent compound Which two solutions will undergo a double replacement reaction and form a white solid substance? NaCl (aq) and LiNO3 (aq) KCl (aq) and AgNO3 (aq) KCl (aq) and LiCl (aq) NaNO3 (aq) and AgNO3 (aq) All of the following are gases. 2C2H2 + 5O2 4CO2 + 2H2O How many grams of oxygen are needed to react completely with .4 moles of ethyne? Cu + 4HNO3 Cu(NO3)2 + 2H20 + 2NO2 Using 32 grams of copper how much of each product is produced ? Answer in grams Calculate the number of molecules in 12 grams of methane and the gas volume it would occupy. Using the combined gas law, what do you have to do to the temperature to double the volume of a gas. 2 liters of He at STP is increased to 27degree C and the pressure decreased to 80kPa. What is new volume? A flask contains 3 gases. Partial pressure of gas A = 30atm, gas B = 20atm, gas c = 60atm. What is the total pressure? The container can hold a pressure of 100atm . What is going to happen? A real gas differs from an ideal gas because a real gas (does/does not) have an attraction toward the other molecules. One mole of any gas has the same number of _________ and occupies the same __________ at STP. What is the concentration of a solution of 12 moles of Iron II Nitrate in 3 liters of solution? Which conducts electricity the best? Gas, liquid, solid, aqueous solution 30ml of .8M HCl is diluted to 70ml. What is the new concentration? What is the pH of a hydrogen ion concentration 3.5 x 10-8 mole per liter? What is the pOH? What is the hydroxide ion concentration of a solution with a pH of 5? Arrange in increasing entropy. Gas, liquid, solid What can a chemist do to increase the rate of a chemical reaction? 50g of water heated from 20degrees c to 45 degrees c. How much heat energy was absorbed? Label the Potential Energy graph Which forms a molecular solid made up of polar molecules? Sodium chloride, helium, diamond, water Given the nuclear equation the letter represents….. 232 90 Th 228 88 Ra + X An electron has a charge identical to what nuclear particle? The half-life of carbon-14 is 5,730 years. How much of a 2 g sample would be left after 17,190 years? Explain nuclear fusion and fission OIL Oxidation is losing e- 4HCl + MnO2 RIG reduction is gaining e- MnCl2 + 2H20 + Cl2 Identify what is being oxidized or reduced What is the % of oxygen in sulfuric acid? What is the % of water in the hydrant Copper II Sulfate 7H20