APES: Miller Review Chapter 3
advertisement

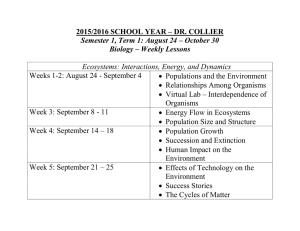
APES: Miller Review Chapter 3 A. Chapter 3: Ecosystems: What Are They and How Do They Work? a. 3-1: How Does the Earth’s Life-Support System Work? i. Earth’s life-support system consists of four main spherical systems that interact with one another. 1. Atmosphere – a thin spherical envelope of gases surrounding the Earth’s surface a. Troposphere – inner thin layer, above sea level at the tropics, contains the air we breath b. Stratosphere – next layer, lower portion contains the ozone (O3) 2. Hydrosphere – made up of all of the water on or near the Earth’s surface a. Water Vapor – found in the atmosphere b. Liquid Water – found on the surface and underground c. Ice – found at the poles, in icebergs, glaciers, ice and permafrost 3. Geosphere – consists of the Earth’s intensely hot core, a thick mantle composed of mostly of rock and a thing outer crust 4. Biosphere – the parts of the atmosphere, hydrosphere, and geosphere where life is found ii. Life depends on three interconnected factors: 1. One-way flow of high-quality energy from the sun, through living things in their feeding interactions, into the environment as low-quality energy and eventually to outer space as heat a. As this solar energy interacts with carbon dioxide and other gases in the troposphere, it warms the troposphere – a process known as the greenhouse effect 2. Cycling of Nutrients – through parts of the biosphere, essentially fixed supply of nutrients must be continually recycled to support life in keeping with the chemical cycling 3. Gravity – allows the planet to hold onto its atmosphere b. 3-2: What are the Major Components of an Ecosystem? i. Ecology – the science that focuses on how organisms interact with one another and with their nonliving environment of matter and energy 1. The study involves interaction among five of these levels: a. Organisms, Population, Communities, Ecosystem, Biosphere 2. Made up of living (biotic) and nonliving (abiotic) factors 3. Each type of organism in an ecosystem is assigned a “feeding level” or trophic level – which depends on its source of food and nutrients a. Producers (Autotrophs) – self-feeders, make the nutrients they need from compounds through photosynthesis i. Carbon Dioxide (CO2) + Water (H2O) + Solar Energy -> Glucose (C6H12O6) + Oxygen (O2) ii. On land most producers are trees and other green plants iii. Freshwater and Ocean ecosystems – algae and aquatic plants growing near shorelines iv. Open water – phytoplankton b. Consumers (Heterotrophs) – other feeders, that cannot produce the nutrients they need through photosynthesis or other processes i. Primary Consumers (Herbivores) – plant eaters ii. Carnivores – meat eaters 1. Secondary Consumers – feed on the flesh of herbivores 2. Tertiary (higher-level) Consumers – feed on the flesh of other carnivores 3. Omnivores – eat plants and other animals 4. Decomposers – consumers that, in the process of obtaining their own nutrients, release nutrients from the wastes or remains of plants, and animals and then return those nutrients to the soil, water, and air for reuse by producers a. Detritus Feeders (Detritivores) – feed on the wastes or dead bodies of other organisms iii. “There is little waste of nutrients in nature” 1. The human species is a major violator of the chemical cycling principal ii. Aerobic Respiration – use oxygen to convert glucose back into carbon dioxide and water 1. Glucose + Oxygen -> Carbon Dioxide + Water + Energy iii. Anaerobic Respiration (Fermentation) – some decomposers get the energy they need by breaking down glucose in the absence of oxygen 1. The end products of this processes are compounds such as methane gas (CH4, the main component of natural gas), ethyl alcohol (C2H6O), acetic acid (C2H4O2, the key component of vinegar), and hydrogen sulfide (H2S, a highly poisonous gas that smells like rotten eggs) a. Only plants carry out photosynthesis iv. Ecosystems and the biosphere are sustained through a combination of one-way energy flow from the sun through these systems and the nutrient cycling of key materials within them c. 3-3: What Happens to Energy in an Ecosystem? i. The chemical energy stored as nutrients in the bodies and wastes of organism flows through ecosystems from one trophic (feeding) level to another. ii. Food Chain – a sequence of organisms, each of which serves as a source of nutrients or energy for the next 1. Primarily through photosynthesis, feeding, and decomposition 2. Use and transfer of energy by organisms involves a loss of some degraded high-quality energy to the environment as heat – 2nd Law of Thermodynamics iii. Food Web – organisms in most ecosystems form a complex network of interconnected food chains iv. Biomass – the dry weight of all organic matter contained in its organisms 1. There is a decrease in the amount of high-quality chemical energy available to organisms at each succeeding feeding level – 2nd Law of Thermodynamics v. Pyramid Energy Flow – illustrates this energy loss for a simple food chain, assuming a 90% energy loss with transfer 1. Too little chemical energy is left after four or five transfers to support organisms feeding at this high trophic levels vi. The amount (or mass) of living organic material (biomass) that a particular ecosystem can support is determined by how much solar energy its producers can capture and store as chemical energy. 1. Gross Primary Productivity – the rate at which an ecosystem’s producers convert solar energy into chemical energy in the form of biomass found in their tissue a. Similar to the rate a which you make money 2. Net Primary Productivity – the rate at which producers use photosynthesis to produce and store chemical energy MINUS the rate at which they use some of this stored chemical energy through aerobic respiration a. Similar to the amount of money you earned per year that you can spend after subtracting your work expenses (transportation, clothes, food, supplies) 3. Despite the low NPP the open ocean produces more of the Earth’s biomass per year than any other ecosystem or life zone 4. Tropical Rain Forest have a very high NPP because they have a large number and variety of producer trees and other plants. 5. The planet’s NPP ultimately limits the number of consumers (including humans) that can survive on Earth d. 3-4: What Happens to Matter in an Ecosystem? i. Biogeochemical or Nutrient Cycles – elements and compounds that make up nutrients move continually through air, water, soil, rock, and living organisms within ecosystems, as well as in the biosphere. These cycles are driven directly or indirectly by incoming solar energy and by the Earth’s gravity. The temporary storage sites such as the atmosphere, the oceans and other bodies of water, and underground deposits are called reservoirs. 1. The Water Cycle (Hydrologic) – collects, purifies, and distributes the Earth’s fixed supply of water, as is powered by energy from the Sun. a. Three major processes – evaporation, precipitation, transpiration b. Surface Runoff – this water flows into streams, which eventually carry water to lakes and oceans c. Aquifers/Groundwater – formations to underground layers of rock, sand, and gravel d. A major medium for transporting nutrients within and between ecosystems. e. The primary sculptor of the Earth’s landscape as it flows over and wears down rock f. Evaporation and subsequent precipitation act as a natural distillation process that removes impurities dissolved in water g. This cycle can be viewed as a cycle of natural renewal of water quality h. Humans alter the water cycle in three major ways: i. Withdraw large quantities of freshwater from rivers, lakes, and aquifers ii. Clear vegetation from land and agriculture, mining, road building, and other activities, and cover much of the land with buildings, concrete, and asphalt. This increases runoff and reduces infiltration iii. Drain and fill wetlands for farming and urban development 2. The Carbon Cycle – The basic building block of the carbohydrates, fats, proteins, DNA, and other organic compounds necessary for life, based on carbon dioxide (CO2). a. Carbon Dioxide (CO2) is a key component of the atmosphere’s thermostat i. If to much is removed the atmosphere will cool ii. If to much is generated the atmosphere will warm b. The linkage between photosynthesis (producers) and aerobic respiration (producers/decomposers/consumers) can circulate carbon in the biosphere c. Marine sediments are the Earth’s largest store of carbon i. High pressure and heat converted them to carbon containing fossil fuels such as coal, oil, and natural gas ii. Long-store carbon was not released to the atmosphere as CO2 until fossil fuels were extracted and burned d. Humans alter the cycle by: i. Mostly adding large amounts of carbon dioxide to the atmosphere when we burn carbon-containing fossil fuels ii. Clearing carbon-absorbing vegetation from forests (carbon footprint) 1. We have a large and growing carbon footprint 3. The Nitrogen Cycle: The major reservoir for nitrogen is the atmosphere, a crucial component of proteins, many vitamins, and nucleic acids such as DNA a. Two natural processes convert, or fix N2, into compounds that plants and animals can use as nutrients i. Electrical Discharge/Lightning ii. Nitrogen-Fixing Bacteria – nitrogen fixation – combine gaseous N2 with hydrogen to make ammonia iii. Ammonia that is not taken up by plants may undergo nitrification 1. Convert most of the NH3 and NH4+ in the soil to nitrate ions (NO3-) – plants then use these forms of nitrogen to produce various amino acids, proteins, nucleic acids, and vitamins iv. Denitrification – bacteria in waterlogged soil and in the bottom sediments of lakes, oceans, swamps, and bogs convert NH3 and NH4+ back into nitrate ions, and then into nitrogen gas (N2), which is released to the atmosphere to begin the nitrogen cycle again. b. Human impact: i. Add large amounts of nitrogen oxides to the atmosphere when we burn gasoline and other fuels and when we use commercial nitrate fertilizers 1. Acid deposition or acid rain ii. The action of anaerobic bacteria on commercial nitrogencontaining fertilizer or organic animal manure applied to the soil iii. Removing large amounts of nitrogen (N2) from the atmosphere faster than the cycle can replace it iv. Industrial processes that convert it to ammonia (NH3) used in fertilizers v. In the aquatic ecosystem by agricultural runoff of fertilizers and animal manure and through discharges from municipal sewage treatment systems. 4. The Phosphorus Cycle: compound of phosphorus (P) circulate through water, the earth’s crust, and living organisms – phosphate ions (PO43-) – DOES NOT INCLUDE THE ATMOSPHERE – major reservoir for phosphorus is the phosphate salts containing PO43- in terrestrial rock formations and oceanbottom sediments a. Running water carries these phosphate ions into the soil where they can be absorbed by the roots of plants and by other producers b. A component of biologically important molecules such as nucleic acids and energy transfer molecules such as ADP and ATP and vertebrate bones and teeth c. The lack of it often limits plant growth on land unless phosphorus is applied to the soil as a fertilizer. d. Also limits the growth of producer populations in many freshwater streams and lakes e. Human Impact: i. The removal of large amounts of phosphate from the earth to make fertilizer ii. Clearing tropical forests, we reduce phosphate levels in tropical soils iii. Phosphate-rich runoff from the land often produces huge populations of algae, which then upset chemical cycling and other processes in bodies of water 5. The Sulfur Cycle – much of the Earth’s sulfur is stored underground in rocks and minerals and in the form of sulfate (SO42-) salts buried deep under ocean sediments a. Also enters the atmosphere from several natural sources i. Active volcanoes and from organic matter broken down by anaerobic decomposers in flooded swamps, bogs, and tidal flats, sea spray, dust storms, and forest fires b. Human Impact: i. We burn sulfur-containing coal and oil to produce electrical power ii. Refine sulfur-containing oil (petroleum) to make gasoline, heating oil, and other useful products iii. Extract metals such as copper, lead, and zinc from sulfurcontaining compounds in rocks that are mined for these materials e. 3-5: How do Scientist Study Ecosystems? i. Both field and laboratory research and mathematical and other models to learn about ecosystems 1. Field Research 2. Remote Sensing 3. Geographic Information System (GIS) 4. Laboratory Research ii. Computer simulations can help scientist understand large and very complex systems, such as lakes, oceans, forests, and the earth’s climate system, that can not be adequately studied and modeled in field and laboratory research 1. Baseline Data – beginning measurements of the variables they are studying in the world’s major terrestrial and aquatic ecosystems a. We need baseline data on the condition of the world’s ecosystems to see how they are changing, to develop effective strategies for preventing or slowing their degradation, and to avoid ecological tipping points beyond which these systems will be disrupted or destroyed.