Matter Study Guide
advertisement

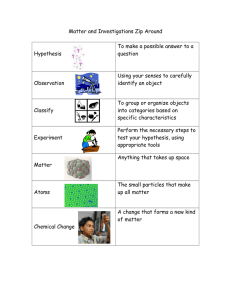
Matter Study Guide Matter is anything that has mass and takes up space. All matter is made up of small particles called atoms. There are three states of matter – solid, liquid and gas. The atoms in a solid are tightly packed together. The atoms in a liquid are loosely connected. The atoms in a gas are not connected. Solids are a type of matter that can’t change its shape or volume. Liquids are a type of matter that can change its shape but not its volume. Gasses are a type of matter that can change its shape and its volume. Matter can change states by adding or taking away heat. Mass is the amount of matter in an object – its weight. Volume is the amount of space matter takes up. Physical properties are anything you can observe using your sense of smell, taste, touch, sight, or hearing. You can observe size, shape, color, texture and flavor. Ex. What can you observe about a penny? Mixtures contain two or more types of matter that can be separated by hand Solutions contain two or more types of matter that can’t be separated by hand Physical change is a change in which no new matter is created. Chemical Change is a change in which new matter is created. Hypothesis is a question that you are investigating. Experiment is the steps you use to test a hypothesis. Observations use your senses to identify objects. Classifications are used to organize objects into categories based on characteristics