BIOL 323– MICROBIOLOGY FALL 2007 Instructor: E-mail:
advertisement

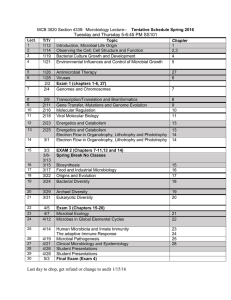
BIOL 323– MICROBIOLOGY FALL 2007 Instructor: Dr. Holly Pinkart Text: Brock Biology of Microorganisms, 11th Ed Lab manual: On Blackboard E-mail: PinkartH@cwu.edu Phone: (509) 963-2710 Office Location: SCI 236D Office Hours: M 2:00-4:00, W 10:00-12:00, or by appointment Course objectives: This is a general course in microbiology. The emphasis is on prokaryotic organisms, with other microbes covered more generally. Students will learn about microbial cell structure, physiology and genetics. These topics will provide a basis for understanding how to control microbial growth, various aspects of metabolism, infectious disease, and microbial ecology. The laboratory component of the course is designed enhance understanding of microbiology through study of microbial cell structure and metabolism. Prerequisites: Cell Biology BIOL 220, and Chemistry through CHEM 113 or CHEM 182/182.1. My Expectations of You: I expect that you will have had the proper prerequisites for this course. This means that you are familiar with basic chemistry (Chapter 3) and the structure of eukaryotic cells (Chapter 4.5-4.7, 14.1-14.5). You will also be familiar with the concept of aerobic cellular respiration; glycolysis, citric acid (aka TCA, Krebs) cycle and the electron transport system in mitochondria. This is a course designed for biology and biochemistry majors; PRE-NURSING AND NUTRITION MAJORS ARE GENERALLY MORE COMFORTABLE IN BIOL 322. I expect academic honesty in all matters (see Appendix B in CWU catalog). Cheating and/or plagiarism will not be tolerated. The following is an outline for the quarter; schedule is subject to change. Topic Introduction Microbial Cell Structure Microbial Nutrition and Metabolism Microbial Growth Control of Microbial Growth Viruses EXAM I Microbial Genetics and Biotechnology Immunology and disease Epidemiology/Infection /Disease Microbial Evolution Microbial Diversity Applied and Industrial Microbiology FINAL EXAM Text Chapter 1,2 4 5, 17 6 20 9 Oct 18, 4:00 pm SCI 328 10, 31 21-22 25-29 11 12- 14 30 Tuesday, Dec 4, 10:00-12:00 Grade Breakdown: Lecture Exam 1 - 20% Final Exam - 20% Quizzes – 10% Grading Scale: 93-100% = A 92-90 = A76-78 = C+ 72-75 = C 60-62 = D<60 = F Lab Practicum 1 – 15% Practicum 2 – 15% Unknown report – 10 % Group presentation (poster) – 10% 86-89 = B+ 69-71 = C- 82-85 = B 66-68 = D+ 79 – 81 = B63-65 = D If you know that you will be absent on an exam date you may take the exam in advance of the test date. Otherwise, if you miss an exam, you must take a comprehensive exam at the end of the quarter to make it up. This applies to one exam only. NO EXCEPTIONS. Quizzes will be posted on BlackBoard on Thursday, and remain available until 11:50 pm Sunday night. Laboratory Schedule: Date 9/20 Description No lab 9/25 9/27 10/2 10/4 10/9 Check in – safety/ Use of Microscope/ Presentations Simple Stains and Gram stain Streaking and inoculation Environmental samples and plate counts, Anaerobic techniques Acid-fast and spore stains 10/11 10/16 10/18 10/23 10/25 10/30 11/1 11/6 11/8 11/13 11/15 11/20 11/27 11/29 Capsule stain and motility Generation time Plaque assay [Lecture Exam 4:00 pm computer lab, SCI 328] Virology, Genome Sequencing & Bioinformatics [meet in computer lab, SCI 328] Practicum I Comparison of antiseptic efficacy, antibiotic action Differential and Selective media Exoenzyme media, Carbohydrate metabolism Nitrogen metabolism and oxygen utilization Differential test patterns, Multiple test media Yogurt production, Unknowns Unknowns; group presentations Unknowns; group presentations Practicum 2, turn in keys Unknown Report due by Monday, Dec 3 @ 5:00PM Some pertinent information and helpful hints: 1) I will use BlackBoard for this course. I will post my Powerpoint presentations there so you will have access to those as well as other course materials. Read the corresponding text chapters prior to class so you can follow course discussions. 2) Please read the lab manual handouts prior to the corresponding lab period. Rewrite your notes soon after class – this will allow you to identify confusing points so you can ask questions. 3) Don’t use your cell phone in lab or during lectures. If you must have them on, set them to vibrate (I understand you may have kids, family issues, etc). Step outside the classroom if it is an emergency. 4) Feel free to ask questions at any time. And please use my office hours! If you cannot make it during my office hours, please make an appointment. 5) Students with disabilities who wish to set up academic adjustments in this class should give me a copy of their “Confirmation of Eligibility for Academic Adjustments” from the Disability Support Services Office as soon as possible so we can meet to discuss how the approved adjustments will be implemented in this class. Students with disabilities without this form should contact the Disability Support Services Office, Bouillon 205 or dssrecept@cwu.edu or 963-2171 ASAP. 6) No, there is no extra credit (except for what is offered on exams). Concentrate on doing well the work that is required, and you won’t need to do extra work to make up for poor performance. COURSE OUTCOMES Microbiological Knowledge Upon completion of this course, students will be able to discuss and explain concepts relating to 3 major themes in microbiology. Theme 1: Microbial cell biology Microbial cellular structure and function Microbial growth and reproduction Cellular energy production and metabolism Structure and reproduction of infectious particles (viruses and prions) Theme 2: Microbial genetics, evolution and diversity Inheritance of genetic information Information flow within a cell Exchange and acquisition of genetic information Causes, consequences and uses of mutations Evolution of microbial genetic and physiological diversity Microbial ecology Theme 3: Interactions and impact of microorganisms and humans Microbial-human relationships Microbial pathogenicity mechanisms Disease transmission Antibiotics and chemotherapy Genetic engineering Biotechnology Laboratory Skills A student successfully completing this course will demonstrate ability to 1. 2. 3. Use a bright field light microscope to view and interpret slides, including a. Correctly setting up and focusing the microscope b. Proper handling, cleaning, and storage of the microscope c. Correct use of all lenses d. Recording microscopic observations Properly prepare slides for microbiological examination, including a. Cleaning and disposing of slides b. Preparing smears from solid and liquid cultures c. Performing wet mount and/or hanging drop preparations d. Performing Gram stains Properly use aseptic techniques for the transfer and handling of microorganisms and instruments, including a. Sterilizing and maintaining sterility of transfer instruments 4. 5. 6. 7. b. Performing aseptic transfer c. Obtaining microbial samples Use appropriate microbiological media and test systems, including a. Isolating colonies and/or plaques b. Maintaining pure cultures c. Using biochemical test media d. Accurately recording macroscopic observations Estimate the number of microbes in a sample using serial dilution techniques, including a. Correctly choosing and using pipettes and pipetting devices b. Correctly spreading diluted samples for counting c. Estimating appropriate dilutions d. Extrapolating plate counts to obtain the correct CFU or PFU in the starting sample Use standard microbiology laboratory equipment correctly, including a. Using the standard metric system for weights, lengths, diameters, and volumes b. Lighting and adjusting a laboratory burner c. Using an incubator Interpersonal and citizenry skills, including a. Working effectively in teams or groups so that the task, results, and analysis are shared b. Effectively managing time and tasks allowing concurrent and/or overlapping tasks to be done simultaneously, by individuals and within a group c. Integrating knowledge and making informed judgments about microbiology in everyday life Laboratory Safety A student successfully completing basic microbiology will demonstrate ability to explain and practice safe 1. Microbiological procedures, including a. Reporting all spills and broken glassware to the instructor and receiving instructions for clean up b. Methods for aseptic transfer c. Minimizing or containing the production of aerosols and describing the hazards associated with aerosols d. Washing hands prior to and following laboratories and at any time contamination is suspected 2. 3. e. Disinfecting lab benches and equipment prior to and at the conclusion of each lab session, using an appropriate disinfectant and allowing a suitable contact time f. Identification and proper disposal of different types of waste g. Reading and signing a laboratory safety agreement indicating that the student has read and understands the safety rules of the laboratory h. Good lab practice, including returning materials to proper locations, proper care and handling of equipment, and keeping the bench top clear of extraneous materials Protective procedures, including a. Tying long hair back, wearing personal protective equipment (eye protection, coats, gloves, closed shoes; glasses may be preferred to contact lenses), and using such equipment in appropriate situations b. Always using appropriate pipetting devices and understanding that mouth pipetting is forbidden c. Never eating or drinking in the laboratory d. Never applying cosmetics, handling contact lenses, or placing objects (fingers, pencils, etc.) in the mouth or touching the face Emergency procedures, including a. Locating and properly using emergency equipment (eye wash stations, first aid kits, fire extinguishers, chemical safety showers, telephones, and emergency numbers) b. Reporting all injuries immediately to the instructor c. Following proper steps in the event of an emergency Always bring a sense of humor to class (the darker the better)!