- Semester 1 Study Guide Chapter 1- Nature of Science Scientific method
advertisement

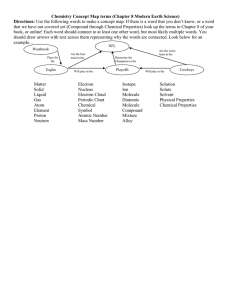
Physical Science- Semester 1 Study Guide Chapter 1- Nature of Science Scientific method hypothesis variable constant control model theory technology volume mass graph experiment dependent variable independent variable scientific law density Chapter 18- Classification of Matter substance element compound solution colloid Tyndell effect physical property distillation physical change law of conservation of mass heterogeneous mixture homogeneous mixture suspension chemical property chemical change Chapter 19- Properties of Atoms & the atom nucleus neutron electron electron cloud valance electron mass number isotope periodic table group period Chapter 22- Chemical chemical formula chemical bond molecule binary compound polyatomic ion Bonds ion ionic bond polar molecule hydrate Chapter 23- Chemical Reactions chemical reaction reactant chemical equation coefficient combustion reaction synthesis single-displacement reaction activation energy catalyst exothermic reaction inhibitor Periodic Table proton quark atomic number average atomic mass electron dot diagram noble gases covalent bond nonpolar molecule oxidation number product balanced chemical equation decomposition reaction double-displacement reaction endothermic reaction rate of reaction Chapter 24- Solutions, Acids, & Bases solution solute aqueous solution solubility unsaturated solution concentration acid indicator strong acid weak acid weak base pH Standards of Measurement kilomilliKelvin conversions derived unit solvent saturated solution supersaturated solution base strong base centiSI system Concepts to Know Steps of the scientific method Converting m to cm; L to mL, Celsius to Kelvin Equation for volume and density What is plotted on the x and y axis Three types of graphs and what information is used with each Benefits of the SI system Examples of physical change, physical property, colloid, suspension How to write a formula with a hydrate Name formulas- What is NaCl? How to write formulas- Write the formula for sodium chloride How to balance equations-H2 + O2 H2O Symbols- s, g, l, aq, How to find group and period of elements Classify elements as metal, nonmetal, or metalloid What to change and not change when balancing equations How H3O units are formed Classify reactions- synthesis, decomposition, single & double displacement 3 factors that affect rate of reaction *What is meant by a balanced chemical equation *Describe an ion and explain how it is used in bonding *Why don’t noble gases form compounds *3 types of subatomic particles-use chart below subatomic location charge mass particle