HEALTH STATE MONITORING SYSTEM DESIGN A Project
advertisement

HEALTH STATE MONITORING SYSTEM DESIGN
A Project
Presented to the faculty of the Department of Electrical and Electronic Engineering
California State University, Sacramento
Submitted in partial satisfaction of
the requirements for the degree of
MASTER OF SCIENCE
in
Electrical and Electronic Engineering
by
Viorel Rotar
FALL
2012
HEALTH STATE MONITORING SYSTEM DESIGN
A Project
by
Viorel Rotar
Approved by:
__________________________________, Committee Chair
Dr. Warren D. Smith
__________________________________, Second Reader
Koullis Pitsillides
____________________________
Date
ii
Student: Viorel Rotar
I certify that this student has met the requirements for format contained in the University
format manual, and that this Project is suitable for shelving in the Library and credit is to
be awarded for the Project.
__________________________, Graduate Coordinator, __________________
Preetham B. Kumar
Date
Department of Electrical and Electronic Engineering
iii
Abstract
of
HEALTH STATE MONITORING SYSTEM DESIGN
by
Viorel Rotar
The health of hospitalized patients often deteriorates, because the available
medical staff is unaware of the deterioration. The condition of normally healthy people
under stressful situations can deteriorate, because they are not aware that they have
reached their physical limits. Infants can die in their “Sleep” because of Sudden Infant
Death Syndrome (SIDS). Death could be prevented by waking the child, but the parents
are unaware that the infant experiences lack of oxygen. People with epilepsy can die
because a seizure was not detected in a timely manner. In severe epileptic episodes, death
from airway constriction can occur in less than 3-5 minutes unless help is immediately
provided.
Therefore, a Health State Monitoring System (HSMS), able to detect and alarm
when a health abnormality develops, is desirable. The HSMS should be broadly useful
from being used by professional health-care providers to personal in home use. It should
be easy to use by first responders, military personnel, firefighters, athletes, and parents
monitoring infants.
iv
The HSMS consists of a wearable device with sensor and communication
circuitry, a personal computer (PC) based graphical user interface (GUI), and a basestation interface between the wireless sensor and the PC. The wearable device
incorporates sensors that can provide a quick general assessment of a person’s cardiorespiratory status, temperature, and level of physical activity. The cardio-respiratory
status is assessed by a pulse oximeter, which gives the percent oxygen saturation in the
blood. Heart rate is derived from the pulsatile waveform of the pulse oximeter which
corresponds to the cardiac cycle. Skin temperature is measured with a digital temperature
sensor. A micro Secure Digital (microSD) card is used to store raw data for extended
periods of time. The sensors for vital data acquisition are non-invasive and do not require
a professional health care provider for attachment to the body. The system is configurable
to accommodate different applications. For continuous patient monitoring, the system can
be configured to wirelessly transmit collected data to a computer. These data then can be
sent to a physician, who then reviews the data and intervenes when necessary. For
applications where a computer is out of wireless communications range, like when
monitoring the effect of stressful situation on health of first responders, the data are saved
on the microSD card for later review. For applications where just an alarm is necessary to
indicate a breathing or heart rate abnormality, such as monitoring of infants for the
detection of SIDS, for example, the system can be configured to activate the light and/or
sound alarm.
v
In this project, a small wearable wireless sensor was designed and built that
incorporates multiple sensors, and all the sensors operated properly in a laboratory
setting. The sensors that are less susceptible to motion artifacts, such as temperature,
were easier to implement. The pulse oximeter performed well under different light
conditions, but data were disrupted or incorrect as a result of motion artifacts.
Development of software algorithms to reduce the effects of motion artifacts is desirable.
The pulse oximeter signal was obtained easily when a finger was placed over the sensor,
but the sensor was not tested at other locations on the body. The wireless communication
was stable, with a range suitable for hospital and home use. The necessity for different
component values for the transceiver, if there is a need to change the frequency band to
comply with local radio-communication rules, is a drawback.
Approved by:
__________________________________, Committee Chair
Dr. Warren D. Smith
____________________________
Date
vi
ACKNOWLEDGEMENTS
It is a pleasure to acknowledge the assistance received during the development of
the Health State Monitoring System from Dr. Warren D. Smith, who is committee chair
for the project, and Koullis Pitsillides, a biomedical engineer from Endosomatic Systems.
vii
TABLE OF CONTENTS
Page
Acknowledgements ........................................................................................................... vii
List of Figures .................................................................................................................... xi
Chapter
1.
INTRODUCTION ........................................................................................................1
2.
BACKGROUND ..........................................................................................................5
2.1.
2.2.
2.3.
Temperature Monitoring ....................................................................................5
2.1.1.
Temperature Monitoring Importance ................................................. 5
2.1.2.
Temperature Measurement Principle ................................................. 6
Activity Monitoring ...........................................................................................8
2.2.1.
Activity Measurement Importance ..................................................... 8
2.2.2.
Activity Measurement Principle ......................................................... 9
Cardio-Respiratory Assessment .......................................................................10
2.3.1.
Pulse Oximeter Importance .............................................................. 10
2.3.2.
Principle of Pulse Oximeter Measurements ..................................... 11
2.3.3.
Optical Properties of the Tissue ....................................................... 12
2.3.4.
Transmission and Reflectance Modes of Pulse Oximeter
Measurements .................................................................................. 18
2.3.5.
3.
Interference to the Pulse Oximeter Signal ........................................ 19
HEALTH STATE MONITOR CIRCUITRY ............................................................21
viii
3.1.
Health State Monitor Circuitry Overview........................................................21
3.2.
Sensor Circuitry ...............................................................................................23
3.3.
3.4.
4.
3.2.1.
Temperature Monitoring Circuitry ................................................... 24
3.2.2.
Activity Monitoring Circuitry .......................................................... 25
3.2.3.
Pulse Oximeter Circuitry .................................................................. 26
Supporting Circuitry ........................................................................................31
3.3.1.
Power Supply Circuitry .................................................................... 31
3.3.2.
Microcontroller (MCU) Circuitry..................................................... 32
3.3.3.
Radio Communication Circuitry ...................................................... 35
3.3.4.
MicroSD Card Circuitry ................................................................... 36
3.3.5.
Alarm Circuitry ................................................................................ 37
Base-Station Circuitry......................................................................................38
WIRELESS SENSOR SOFTWARE ..........................................................................39
4.1.
Microcontroller Main Routine .........................................................................39
4.2.
Temperature Acquisition .................................................................................42
4.3.
Accelerometer Acquisition ..............................................................................43
4.4.
Pulse Oximeter Acquisition .............................................................................44
4.4.1.
Pulse Oximeter Software Overview ................................................. 44
4.4.2.
Controlling the LEDs and Taking Data Samples ............................. 46
4.4.3.
Red and Infrared (IR) Signal Processing .......................................... 49
4.4.4.
Calculating the Heartbeats ................................................................ 52
ix
4.4.5.
5.
Calculating the Percent Oxygen Saturation in Blood ....................... 55
4.1.
MicroSD Card Software ..................................................................................58
4.2.
Wireless Communication .................................................................................61
GRAPHICAL USER INTERFACE (GUI) ................................................................63
5.1.
Initializing a Wireless Sensor ..........................................................................63
5.2.
Controlling a Wireless Sensor .........................................................................63
5.3.
Displaying Sensor Data....................................................................................66
5.4.
Saving Sensor Data ..........................................................................................66
6.
BASE STATION INTERFACE SOFTWARE ..........................................................69
7.
TESTING RESULTS .................................................................................................70
8.
SUMMARY, CONCLUSIONS, AND RECOMMENDATIONS .............................73
References ..........................................................................................................................77
x
LIST OF FIGURES
Figures
Page
1.
Figure 2.1. Circadian temperature rhythm. ......................................................... 5
2.
Figure 2.2. Temperature measurement variation with location. ......................... 7
3.
Figure 2.3. Data from a daily routine activity monitor. ...................................... 9
4.
Figure 2.4. Laser light penetration. .................................................................... 13
5.
Figure 2.5. Tissue light absorption. .................................................................. 14
6.
Figure 2.6. Absorption of oxygenated and deoxygenated haemoglobin versus
wavelength...................................................................................... 15
7.
Figure 2.7. Ratio of red to infrared amplitudes versus blood oxygenation....... 16
8.
Figure 2.8. Dependence of light absorption on tissue. ...................................... 17
9.
Figure 2.9. Dependence of blood cell alignment on flow. ................................ 17
10.
Figure 2.10. Systole and diastole correlation to signal. .................................... 18
11.
Figure 2.11. Transmission mode (left) and reflectance mode (right) ............... 19
12.
Figure 2.12. Normal and undesirable pulse oximeter signals. .......................... 20
13.
Figure 3.1. Wireless sensor schematic. ............................................................. 22
14.
Figure 3.2. Base-station schematic ................................................................... 23
15.
Figure 3.3. Temperature sensor circuitry .......................................................... 24
16.
Figure 3.4. Accelerometer circuitry .................................................................. 25
17.
Figure 3.5. Wireless sensor electronic assembly .............................................. 26
xi
18.
Figure 3.6. Red and IR light source. ................................................................. 27
19.
Figure 3.7. Photodetector and amplification circuitry ...................................... 28
20.
Figure 3.8. Relative intensity versus wavelength. ............................................ 29
21.
Figure 3.9. Photodiode relative spectral sensitivity versus wavelength ........... 30
22.
Figure 3.10. Power supply circuit ..................................................................... 32
23.
Figure 3.11. Microcontroller circuit.................................................................. 35
24.
Figure 3.12 RF circuit ....................................................................................... 36
25.
Figure 3.13. MicroSD card circuit .................................................................... 37
26.
Figure 3.14. Sound and light alarm circuit ....................................................... 38
27.
Figure 4.1. Wireless sensor initialization .......................................................... 39
28.
Figure 4.2. Wireless sensor microcontroller ..................................................... 40
29.
Figure 4.3. IR (bottom) and red (top) LEDs on/off sequence ........................... 47
30.
Figure 4.4 First stage amplifier settling time .................................................... 47
31.
Figure 4.5. First (top) and second (bottom) stage amplifier outputs ................ 48
32.
Figure 4.6. Displaying unfiltered red and filtered IR signals ........................... 51
33.
Figure 4.7. Pulse detecting signal (on T channel) ............................................. 52
34.
Figure 4.8. IR heart pulse (top) and pulse detect (bottom) ............................... 54
35.
Figure 4.9. The ac and dc components of the IR signal. ................................... 56
36.
Figure 4.10. Percent oxygen saturation versus R/IR ratio. ............................... 57
37.
Figure 4.11. Pulse oximeter function ................................................................ 59
38.
Figure 4.12. MicroSD card function ................................................................. 60
xii
39.
Figure 5.1. GUI timed acquisition control panel .............................................. 64
40.
Figure 5.2. Wireless sensors grouped for timed acquisition ............................. 65
41.
Figure 5.3. Wireless sensors grouped for timed acquisition for different
On/Sleep times ............................................................................... 65
42.
Figure 5.4. Display monitor. ............................................................................. 67
43.
Figure 5.5. Plotting saved data in Excel. .......................................................... 68
44.
Figure 7.1. Accelerometer data (three axis) ...................................................... 72
xiii
1
CHAPTER 1
1. INTRODUCTION
Development of continuous remote health monitoring is necessary, because
people live longer and “baby boomers” are aging, threatening to overwhelm health-care
providers [1]. Implementation of continuous health monitoring contributes to an increase
in health-care quality and a decrease in health-care cost. Prevention of the onset of
chronic disease can dramatically reduce hospitalization time and increase the quality of
life, with the associated reduction of health care costs.
A person’s health can drastically worsen between scheduled physician visits. But,
with continuous health state monitoring, office visits can be reduced, because a physician
reviews collected data more frequently. Continuous remote health monitoring will enable
aging people and people with chronic illnesses to live in their homes and be independent.
A continuous health monitoring system will aid people with epilepsy. The
standardized mortality rate (SMR) is 1.6-9.3 times higher for people with epilepsy than in
the healthy population [2]. In severe epileptic episodes, death from airway constriction
can occur in less than 3-5 minutes unless help is immediately provided. Detecting
changes in heart rate and oxygen concentration in the blood would indicate that the
person is in crisis or that a crisis is imminent and help is needed.
Continuous monitoring aids in detecting changes in health condition in postoperative hospitalized patients. A post-operational patient monitoring system for
detecting deterioration in post-operative hospitalized patients, where medical staff is
2
available but is unaware of the deterioration, has proven very useful [3]. The monitoring
system consisted of pulse oximeters worn by the patients that notified a nurse via wireless
pager when abnormalities were detected. The system helped doctors to intervene before a
critical situations developed. According to the results of the study, rescue events
decreased from 3.4 to 1.2 per 1,000 patient discharges and intensive care unit transfers
from 5.6 to 2.9 per 1,000 patient days. Pulse-oximeter implementation saved the 36-bed
intensive care unit 135 days per year.
In the United States, about 2,500 infants die each year because of SIDS [4].
Apparently, the brain triggers the babies to wake from sleep and cry when a lack of
oxygen is detected. This response changes their heartbeat and breathing patterns to make
up for the lowered oxygen and excess carbon dioxide. If this mechanism is not
developed, the infants die in their sleep. A continuous monitoring system, capable of
measuring heart rate and oxygen concentration in blood, could prevent many infant
deaths.
Continuous health monitoring could be used for healthy people who are in
stressful situations, such as firefighters, combat personnel, and athletes. The development
and initial bench testing of a continuous monitoring system using a pulse oximeter and an
accelerometer has been done for such applications [5]. The system was used for
monitoring first responders and critically injured persons. The oxygen concentration and
heart rate gives the health state of a soldier, and the accelerometer shows the soldier’s
activity and body orientation.
3
An internet-based health monitoring system has been developed and tested for
monitoring brain-injured children [6]. The test results showed that even though the
system needs improvements, 78% of patients and families were interested in further use
of the system.
Continuous health monitoring will play a big role in the health-care industry and,
according to Kalorama, the market will increase from $ 5.7 billion in 2009, with an
annual growth of around 26 % through 2014 [7]. General Electric and Intel joined to
create a health-care company to develop telehealth systems [8].
The goal of this project is to develop a remote continuous noninvasive Health
State Monitoring System (HSMS), capable of indirect measurement of the percent
oxygen saturation in the blood, heart rate, physical activity, and temperature. The system
would consist of a wearable device with a unique identification number (ID), containing
sensors and communicating circuitry, called further the “wireless sensor,” a personal
computer (PC) based graphical user interface (GUI), and a base-station interface between
the wireless sensor and the PC. The wireless sensor, with a unique ID, consists of a pulse
oximeter, activity and temperature sensors, wireless circuitry for communication with the
base-station, a programmable microcontroller, local data storage, and local sound/light
alarms.
The GUI allows the user to remotely access an individual ID addressable wireless
sensor for controlling the wireless sensor and for retrieving the sensor data in real time.
The control involves setting the sensor’s data acquisition rate, enabling/disabling the
sensors, enabling/disabling data saving on the wireless sensor microSD card, and setting
4
the source and levels for triggering sound and/or light alarms. The base-station is an
interface between the PC and the wireless sensor that allows the wireless sensor to send
data to the PC and the PC to control the wireless sensor.
This report is structured as follows: Chapter 2 provides a background description
of the sensors for collecting vital data used in the project. The description includes the
importance of the sensor measurement, application areas, and the potential for expanding
the application areas. Chapter 3 describes the circuit that was built for this project. The
circuit description is divided into two main sections, the sensor circuitry and supporting
circuitry. The sensor circuitry section describes the circuits and hardware of the
temperature, activity, and pulse oximeter sensors. The core components of each sensor
are described. The supporting circuitry section describes the circuit and the hardware of
the power supply, wireless communication, microcontroller features, microSD storage,
the sound and light alarm, and the microcontroller that controls the hardware. Chapters 4,
5, and 6 describe the software and provide software flowcharts and code snippets for the
HSMS. There are three independent units: the wireless sensor software is described in
Chapter 4, the GUI software is described in Chapter 5, and the base-station software is
described in Chapter 6. Test results for the HSMS are provided in Chapter 7. Chapter 8
gives a summary, conclusions, and recommendations for improving the system.
5
CHAPTER 2
2. BACKGROUND
2.1. Temperature Monitoring
2.1.1. Temperature Monitoring Importance
Temperature measurement commonly is used to determine if a person has a fever
and, if so, the severity of the fever. However, body temperature also is affected by
stressful situations, a person’s age and activity, time of the day and season, pregnancy,
hormonal contraceptives, etc. The temperature of the human body has a twenty-four hour
pattern (see Figure 2.1), called a circadian rhythm, and a season pattern, called a
circannual rhythm [9].
Figure 2.1. Circadian temperature rhythm.
The figure show 24-hour temperature variation for various location [9].
6
Continuous temperature measurement can provide additional information about a
person’s state of health. For example, the disruption in timing of acrophase (the time at
which the peak of a rhythm occurs) is associated with insomnia and several chronic
diseases, including cancer and HIV. Continuous temperature measurement not only can
be used for detecting medical abnormalities but also for evaluating the effectiveness of
medications.
2.1.2. Temperature Measurement Principle
The importance of clinical temperature measurement is traced back to the times of
Hippocrates [10]. At that time, hands were used to estimate the heat or cold of a human
body. The first physician to use a thermometer at his patients' bedside is considered to be
Hermann Boerhaave in the 16th century. Sir Thomas Allbutt invented in 1867 the first
practical medical thermometer used for taking the temperature. It was portable, 6 inches
in length, and able to record a patient's temperature in 5 min. Many devices were
developed for temperature measurement. One of the most common devices for measuring
temperature is the glass thermometer which uses the thermal expansion properties of a
liquid (spirits, mercury). Modern thermometers are based on semiconductor temperature
sensors and are capable of taking a measurement in 5 to 10 s. Temperature sensors are
available from different manufacturers. A sensor needs to be selected based on size,
accuracy, type of output (analog or digital), power consumption, sensor price, and, for
digital sensors, the choice of I2C or SPI communication protocol.
7
For this project, a low-cost temperature sensor, the TMP121 from Texas
Instruments (TI), Dallas, Texas, with ± 1.5 °C accuracy and with the 0.0625 °C resolution
was selected. For continuous monitoring, body tracking temperature variation is more
important than accuracy. Body temperature varies based on gender and on different
locations on the body (Figure 2.2) and depends on the season, time of the day, and the
person’s fitness [9]. The body temperature measurement is done every 30 s (default
setting), because body temperature changes relatively slowly.
Figure 2.2. Temperature measurement variation with location.
The figure shows the temperature measurement variation in dependence of gender and
measurement location [9].
8
2.2. Activity Monitoring
2.2.1. Activity Measurement Importance
Health is in direct relationship with a person’s lifestyle. Many health problems
could be prevented by sufficient physical activity and by sufficient rest. Staying “in
shape” requires exercise [11]. But, over-exercising harms health. For example, “intense
exercise can cause scarring and fibrosis in the heart” [12]; vigorous activity can “increase
the risk of sudden cardiac death and acute myocardial infarction” [13]. Insufficient as
well as excessive [14] sleep can cause cardiovascular diseases [15], diabetes, mental
problems [16], [17], and accidents [18]. Therefore, by monitoring activity, in correlation
with other physiological measurements, a safe exercise intensity and rest time program
can be set. For example, cardiac infarction can occur during jogging. By correlating the
occurrence of heart abnormalities with activity intensity, a safe level of activity intensity
can be determined. Exceeding the set activity level would trigger an alarm, warning the
jogger to decrease the activity. By analyzing the rest time (sleep, watching TV etc.) of a
person, a correction to the lifestyle can be suggested. Figure 2.3 shows an example of
data from a daily routine activity monitoring [19].
A decrease in activity during the day or increase in activity during the night, in
comparison with the previously collected data for a specific person, can indicate that the
person experiences some discomfort. For example, a person suffering from pain may
reduce daily activities. At the same time, because of the pain, the person might not sleep
well, resulting in an increase in activity during the rest period.
9
Figure 2.3. Data from a daily routine activity monitor.
The figure shows three-axes accelerometer data in dependence of the persons activity.
The standing, sitting, walking, lying and running activities are detected.
Activity monitoring can be used for fall detection [20], balance control
evaluation, and determining walking [21]. Falling is one of the major causes of injuries
for elderly, and even if diligent efforts are made to prevent elderly, falls are often
inevitable [20]. Fall detection can alert that a person has fallen and needs help.
2.2.2. Activity Measurement Principle
For the project, a three-axis accelerometer is used for continuous activity
monitoring. The accelerometer is capable of detecting six directions of movement and
10
can accurately detect gravity. Therefore, activities such as running, standing, and lying
can be determined. The accelerometer can be set to generate an interrupt if the activity
decreases below a set threshold or when activity exceeds a set threshold for a user set
time duration. The variability in daily activity of a person can be used to estimate a
person’s health.
2.3. Cardio-Respiratory Assessment
2.3.1. Pulse Oximeter Importance
A pulse oximeter measures the percent oxygen saturation in the blood. Because
the pulse oximeter quickly gives a general assessment of a patient's cardio-respiratory
status, and the measurement is non-invasive, the pulse oximeter has become a standard
monitoring device before, during, and after operations [22]. The device also is used in
intensive care, recovery rooms, and in emergencies [23].
Existing pulse oximeters, due to their high power consumption, are not intended
for long term monitoring and are usually attached to a finger, an earlobe, or the forehead.
Using a pulse oximeter for long term monitoring allows determining the heart rate
variability (HRV). The importance of HRV is that it can be used to predict potential
complications in pregnancies, fetal distress, and in neonatal critical care [24]. The HRV
can be used for assessing the severity of congestive heart failure [25], stroke, Parkinson’s
disease and depression for people with chronic disorders [26].
11
2.3.2. Principle of Pulse Oximeter Measurements
Blood oxygen concentration, pulse, and pressure show the function of the heart
and lungs. Pulse oximeter devices use (at least) two sources of light of different
wavelengths and use the fact that oxygen saturated blood and unsaturated blood absorb
the light differently.
The oxygen in the body is carried by the protein hemoglobin (Hb) in the red blood
cells. The oxygen is bound to hemoglobin in the lungs and then transported through the
entire organism for respiratory processes. Oxygen saturation (SO2) is defined as the ratio
of the concentration of oxygenated hemoglobin (HbO2) to the total concentration of
hemoglobin. This is,
SO2
CHbO2
CHbO2 CHb
,
(0.1)
where C is concentration and Hb is deoxygenated hemoglobin.
Using two light sources with such wavelengths that one is absorbed more by the
oxygenated hemoglobin and another more by the unoxygenated hemoglobin allows
determining the blood oxygen saturation. The relevant parameter for estimating the blood
oxygen concentration is the light intensity, which is affected by absorption, and is
measured by a photosensor. The signal detected by the photodetector consists of ac and
dc parts. The ac part corresponds to the light absorption by the blood cells, and the dc
part corresponds to the light absorption by the tissue. The dc part corresponds to the light
12
absorption by the tissue because the consistency of the tissue (water, melanin) changes
very slowly. Because the light absorption by blood cells varies as a result of blood
pulsation, it results in the ac part of the detected signal. The ratio of ratios formula that is
needed for calculating the blood oxygen saturation is
I max Red
)
I min Red
R
,
I
ln( max IRed )
I min IRed
ln(
(0.2)
where I min Red and Imin IRed are the minimum values of the ac components of the red and
infrared light sources, respectively, and Imax Red and I max IRed are the maximum values of
the ac components of the red and infrared light sources, respectively. The ratio of
logarithmic normalized intensities is used to calculate the oxygen concentration using an
empirical calculated calibration curve.
2.3.3. Optical Properties of the Tissue
The tissue is made up of cells of different functions and sizes; therefore, the tissue
is an inhomogeneous medium with different and randomly distributed absorbers and
scatterers. When the tissue is irradiated by light, photo-thermal and photochemical
reactions occur, including fluorescence, optical reflectance, and transmission processes.
The absorption of light through the tissue is wavelength dependent; therefore, the
penetration depth of light in tissue is different for different wavelengths (see Figure 2.4)
13
[4]. The peak depth penetration of 1.4 mm is at 1064 nm. Light penetration depth is
defined as the depth where the incident irradiance of the light is decreased to 37 % of the
original intensity.
KrF XeCl Dye Argon Diode Nd:YAG Tm:YAG Ho:YAG Er:YAG CO2
248 308 465 514.5 830
1064
2010
2100
2940
10600 λ (nm)
1 µm
5µm
20 µm
40 µm
150 µm
250 µm
330 µm
300-400 µm
1300 µm
1400 µm
Figure 2.4. Laser light penetration.
The figure shows light penetration depth in tissue the dependence of laser light
wavelength (adapted from [27]).
Figure 2.5 shows plots of absorption coefficients versus wavelength for protein,
melanin, collagen, water, deoxygenated hemoglobin, and oxygenated hemoglobin - the
main components of the tissue. At wavelengths lower than the 805 nm isosbestic point,
14
Figure 2.5. Tissue light absorption.
The figure shows the light absorption coefficients for protein, melanin, collagen, water,
deoxygenated hemoglobin, and oxygenated hemoglobin versus wavelength [28].
Hb is the strongest absorbent, and at wavelengths higher than 805 nm, HbO2 is the
strongest absorbent. In the range from 600 nm to 1000 nm, the deoxygenated and
oxygenated hemoglobin are the main light absorbents, and this range is called the tissue
window. The range of the tissue window and the absorption coefficients of Hb and HbO2
in this window dictate the wavelengths used for a pulse oximeter. In this tissue window,
660 nm, created by a red light emitting diode (LED), and 940 nm, created by an infrared
LED, wavelengths are chosen. The absorptions of the red and infrared signals are not
equal, as seen in Figure 2.6. Therefore, different signal amplitudes are expected for red
and infrared LEDs having equal light intensities, and the changes in signal amplitude
15
Figure 2.6. Absorption of oxygenated and deoxygenated haemoglobin versus
wavelength.
The figure shows the relative light absorbtion levels of oxygenated and deoxygenated
blood versus wavelength [29]. The light absorption levels for the red and infrared are
marked. The isobestic point (light absorbtion coefficients of oxygenated and
deoxygenated blood are equal) is at 805 nm and cannot be used for pulse oximetry.
received from red and infrared LEDs are different for different levels of oxygen
concentration in blood. For saturations below 85 %, the signal from the red LED has the
largest ac part and for oxygen saturations higher than 85 %, the infrared (IR) signal has
the largest ac part. At 85 % oxygen saturation, the ratios of the red to the IR ac
components of the signals equals one, and at 100 %, the signals’ ac ratio is 0.43 [6].
16
Figure 2.7 [30] shows the ratio of the red to infrared signal amplitudes for different
percentage oxygen saturation in blood.
Figure 2.7. Ratio of red to infrared amplitudes versus blood oxygenation.
The figure shows that the amplitude of the red signal decreases (absorption increases) and
the amplitude of the infrared signal increases (absorption decreases) with the increase of
oxygenation [30].
Equation (0.1) shows the oxygen saturation determined by a pulse oximeter. The
measurement is actually called the functional oxygen concentration, because there are
other types of hemoglobin in blood, such as carboxyhemoglobin (COHb) and
methemoglobin (MetHb). The dependence of light absorption on tissue is shown in
Figure 2.8 [28]. The light absorption by the tissue, venous blood, and nonpulsating
arterial blood in the path of the light constitutes the constant portion of the signal.
17
Figure 2.8. Dependence of light absorption on tissue.
The figure shows that the ac component of light absorption is created by the pulsating
arterial blood and the dc component is created by nonpulsating tissue [28].
Figure 2.9 [29] shows that the ac portion is affected by the changes in the orientation of
the blood versus blood flow rate. During systole (maximum), the blood cells, which have
the form of a biconcave disk, are aligned perpendicular to the blood flow, increasing light
absorption, and during diastole (minimum), the blood cells are aligned parallel to the
flow (see Figure 2.10 [31]).
Figure 2.9. Dependence of blood cell alignment on flow.
The figure shows changes in blood cell alignment during and in between heartbeats [29].
18
Figure 2.10. Systole and diastole correlation to signal.
The figure shows blood cell alignment at systole and diastole and correlation to the
detected signal [27].
2.3.4. Transmission and Reflectance Modes of Pulse Oximeter Measurements
Transmission and reflectance type of pulse oximeters use the same technology.
The difference is in the photodetector and light sources relative placement.
In transmission mode, the light source and the photodetector are placed on
opposite sides of the investigated object. This method is more common and is used in
hospitals where a sensor-clip is attached to the person’s finger or ear lobe.
19
In reflective mode, the light sources and the photodetector are placed on the same
surface. This configuration allows making smaller patch type sensors, which also have
less restriction on body placement. Figure 2.11 shows the transmission mode and
reflectance mode principle.
Figure 2.11. Transmission mode (left) and reflectance mode (right).
The figure shows the position of the photodetector relative to the light sources for
transmission and reflective types of pulse oximeters [32].
2.3.5. Interference to the Pulse Oximeter Signal
The pulse oximeter signal is degraded by the low perfusion, noise artifacts, and
motion artifacts. Figure 2.12 [30] shows the shape of a normal pulse oximeter signal (top)
and degraded pulse oximeter signals. Noise artifacts are created by electrical sources.
High frequency noise can be reduced by low pass filtering. To reduce low frequency
noise, the signal can be averaged over multiple pulses.
20
Figure 2.12. Normal and undesirable pulse oximeter signals.
The figure shows the waveforms obtained from a pulse oximeter. The shape of a normal
signal is the desired signal. Low perfusion is caused by health conditions (cardiac
arrhythmias, heart failure, peripheral vascular disease, hypotension) [33]. Noise artifacts
are caused by electrical equipment. Motion artifacts are caused by the movments of the
person using the pulse oximeter.
21
CHAPTER 3
3. HEALTH STATE MONITOR CIRCUITRY
3.1. Health State Monitor Circuitry Overview
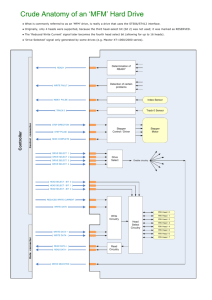
The HSMS circuitry consists of the wireless sensor circuitry and base-station
circuitry. An overview of the wireless sensor circuitry is shown in Figure 3.1 and consists
of
1.
Sensor circuitry
2.
Temperature sensor circuitry (TEMP)
3.
Accelerometer circuitry (ACCEL)
4.
Pulse oximeter circuitry that consist of photodetector (AMP) and light
generation and control (IR_RED) circuitries
5.
Supporting circuitry
6.
Power supply circuitry (PS) for controlling the power supply voltage
7.
Microcontroller (MCU) circuitry that controls all the circuits
8.
Wireless communication circuitry (RF)
9.
Local data storage circuitry (microSD)
10.
Light and sound (ALARM) alarm circuitry.
22
Figure 3.1. Wireless sensor schematic
An overview of the base-station circuitry is shown in Figure 3.2 and consists of
1.
Power supply circuitry (PS) for controlling the power supply voltage
23
2.
Microcontroller circuitry (MCU) that controls all the circuits
3.
Wireless communication circuitry (RF)
4.
Serial communication.
Figure 3.2. Base-station schematic
3.2. Sensor Circuitry
This section describes the circuitry of the sensors that are used for determining
health status data. It includes temperature, activity, and pulse oximeter circuitry. For
measuring temperature and activity, dedicated integrated sensors are used.
24
3.2.1. Temperature Monitoring Circuitry
For temperature measurement, a dedicated integrated sensor, the TMP121 is used.
It has 12 bits of resolution plus one bit for indicating negative temperatures. The sensor
has 0.0625 °C resolution and ± 1.5 °C accuracy in the −25 °C to +85 °C temperature
range. In the anticipated temperature range, based on circadian rhythm (see Figure 3.3),
the accuracy is -0.5, +0.9 °C. The temperature sensor is accessed via a serial peripheral
interface (SPI), acquires a new temperature measurement every 0.5 s and needs 0.25 s to
perform the conversion. The sensor has the following features:
1.
Power supply range: 2.7 V to 5.5 V
2.
Current Consumption
a. Temperature acquisition - 50 µA
b. Idle state - 20 µA
c. SPI access - unspecified
Figure 3.3. Temperature sensor circuitry
25
3.2.2. Activity Monitoring Circuitry
For activity measurement, an integrated three-axis 12-bit resolution
accelerometer, the LIS331DLH from STMicroelectronics (Geneva, Switzerland) is used.
Accelerometer circuitry is shown in Figure 3.4. The sensor has dynamically selectable
full-scale ranges of ±2 g, ±4 g, and ±8 g, six direction detection, and a data rate of up to 1
kHz. From the available accelerometer options, of interest are low (lower than set
threshold value) and high (higher than set threshold value) interrupts and, depending on
the application, six direction detection.
Figure 3.4. Accelerometer circuitry
Low activity interrupts combined with the time period of low activity are useful
for detecting lower than usual activity for a certain individual or for detecting no activity
26
at all, as when the individual is unconscious. High level activity interrupts are useful for
detecting falls or discomfort. For example, higher than usual activity for a specific
individual during the rest period can indicate that the person feels some discomfort and
needs assistance.
3.2.3. Pulse Oximeter Circuitry
The pulse oximeter circuitry was designed specifically for this project with low
power consumption and small size (see Figure 3.5) as the primary criteria. The pulse
Figure 3.5. Wireless sensor electronic assembly
27
oximeter circuitry consists of red and IR light-emitters (Figure 3.6), and photodetector
and amplifier circuitry (see Figure 3.7). The microcontroller controls the light-emitting
circuitry. It powers sequentially each LED with such a voltage that the LED generates
strong enough light for the photodetector to detect the light reflected by blood cells, but
not too strong to exceed the amplifier U7 (see Figure 3.7) output range and the
microcontroller’s analog-to-digital converter (ADC) input range.
Figure 3.6. Red and IR light source.
The red and IR LEDs’ anodes are connected to individual pins of the
microcontroller, with which the microcontroller controls each LED. The cathodes of the
LEDs are connected to the digital-to-analog converter (DAC) microcontroller pin, via
which the microcontroller adjusts the intensity of the LEDs. The red and the IR LEDs
require different voltage levels to generate equal light intensities. In addition, the
photodetector sensitivity is different for red and infrared lights. Plots of light intensity
Figure 3.7. Photodetector and amplification circuitry
28
29
versus wavelength for the LEDs are shown in Figure 3.8 [34], and the plot of
photodetector sensitivity versus wavelength is shown in Figure 3.9 [35]. The necessary
voltages for the LEDs are calculated and are provided by the DAC. A specialized part,
the SML12R3KIR941T LED from Ledtronics (Torrance, CA), that contains red and
infrared LEDs in one package is used as the light emitting source.
Figure 3.8. Relative intensity versus wavelength.
The figure shows the relative light intensity of the red LED (top), and infrared LED
(bottom) of the light source (LIS331DLH) used in this project [34]
30
Figure 3.9. Photodiode relative spectral sensitivity versus wavelength.
The figure shows the VBPW34S (Vishay Semiconductors, Malvern, PA) photodetector
relative sensitivity versus wavelength [35].
If the wireless sensor is on, then every 10 ms, the microcontroller performs the
following sequence:
1.
turns infrared LED on
2.
waits 500 µs
3.
ADC samples amplifier U7 (see Figure 3.6) output
4.
turns infrared LED off
5.
turns red LED on
6.
waits 500 µs
7.
ADC samples amplifier U7 (see Figure 3.6) output
8.
Turns red LED off.
31
The photodetector circuit consists of a photodiode and a two-stage amplifier. The
first stage is an OPA381 transimpedance amplifier from TI. The second stage is an
OPA333 op-amp, also from TI. The output from the first stage is sampled and is used for
calculating the intensity of the LEDs. Another microcontroller DAC provides an
adjustable voltage to the non-inverting input of the second stage amplifier for removing
the dc component. The microcontroller samples the second stage amplifier output, which
is the signal for calculating the heartbeats and the percent oxygen saturation in blood.
3.3. Supporting Circuitry
This section describes wireless sensor supporting circuitry. It includes power
supply, microcontroller, wireless communication, microSD card, and sound and light
alarm circuitry.
3.3.1. Power Supply Circuitry
The power supply circuit consists of an LM3670 dc-to-dc adjustable converter
from TI. The circuit is shown in Figure 3.10. It provides 3.3 V or 1.8 V. In the active
mode, the power supply voltage is set to 3.3 V. In “Sleep” mode, the output is 1.8 V and
is set by connecting PS_REG to ground. In active mode, the microcontroller switches the
pin to high input impedance.
32
Figure 3.10. Power supply circuit
3.3.2. Microcontroller (MCU) Circuitry
An ATXMEGA32A4 microcontroller from Atmel (San Jose, CA) is used in this
project. The microcontroller circuit is shown in Figure 3.11. It was chosen because of the
following features:
1.
Five “Sleep” modes.
2.
16-bit Real Time Counter (RTC) with a separate oscillator, which runs at 38 kHz.
From the microcontroller’s “Sleep” mode options, the power-save option is
chosen. In this mode, the microcontroller has the lowest power consumption that
has the RTC running. When the wireless device in active mode, the RTC wakes
up the microcontroller from the power-save mode to start data acquisition every
10 ms. When the wireless device is in “Sleep” mode, the RTC wakes the
microcontroller every 2 s.
33
3.
One twelve-channel, 12-bit, 2 Msps ADC. It is possible to configure the ADC to
have up to four differential inputs with or without gain. The gain is software
selectable from 1 x, 2 x, 4 x, 8 x, 16 x, 32 x or 64 x. For this project, the ADC is
configured to have one differential input with gain and one single-ended input.
The differential input is used for acquiring the signal from the photodetector’s
second stage amplifier. The single-ended input is used to acquire the signal from
the photodetector’s first stage, which is used for calculating the LEDs’ light
intensities.
4.
Two-channel, 12-bit, 1 Msps DAC. One channel is used to adjust the LEDs
intensities, and another for adjusting the second stage amplifier dc offset.
5.
Multiple options for ADC and DAC reference. According to the datasheet, the
ADC can use the internal 1 V, internal Vcc/1.6, or external voltage reference on
the AREFF pin as a reference voltage. The DAC has an internal 1 V, external
AVcc, or AREFF reference voltage. In this project, for the DAC, AVcc is the
reference, and for the ADC, Vcc/1.6, which is 2 V for a 3.3 V supply, is the
reference.
6.
Five 16-bit Timer/Counters. Only one timer is used to handle all needed delays.
For example, it handles the 500-µs delay needed for each LED. The wireless
transceiver uses various delays for tune-up and data transmission. During the
delays, the microcontroller is in the idle power saving mode. The timer interrupt
wakes the microcontroller after each delay.
34
7.
Two SPIs. Only one SPI is used to handle the wireless transceiver, accelerometer,
temperature sensor, and the microSD card communications.
8.
Multiple interrupt options for I/O pins. Only one interrupt is used to handle all the
transceiver requests.
9.
Thirty four programmable input/output (I/O) pins. From 34 pins, 30 are
connected. The rest have important functions but were difficult to access. This
microcontroller has a 49-ball VFBGA package. Accessing these pins requires
micro-vias that would increase the cost of the printed circuit board (PCB). Two
connected I/Os also are not used. There are two pins for controlling the power
supply voltage, but in this design, only one is used for switching between 1.8 and
3.3 V. The pin connected to the microSD card detect also is not used.
10.
Multiple dynamically selected clock options. To minimize the wireless sensor
size, only the internal microcontroller clock sources are of the interest. The
internal clock options are: 32-MHz run-time calibrated RC oscillator, 2-MHz runtime calibrated RC oscillator, 32.768-kHz calibrated RC oscillator, 32-kHz Ultra
Low Power (ULP) oscillator with 1-kHz output, and a phase lock loop (PLL) with
1 to 31 x multiplication. The microcontroller always starts at 2 MHz after reset.
For all microcontroller processes, an 8-MHz (2 MHz clock · 4PLL) clock is
selected.
11.
Low power supply voltage. The microcontroller works in the 1.6-3.6 V range.
12.
Small 5 x 5 mm package size.
35
Figure 3.11. Microcontroller circuit
3.3.3. Radio Communication Circuitry
For the wireless communications, the SI4432, a highly integrated single chip
transceiver from Silicon Labs (Austin, Texas) is used. The schematic is shown Figure
3.12. This transceiver covers frequencies from 240–960 MHz in 156-Hz or 312-Hz steps,
has high (–121 dBm) sensitivity, and has seven power transmission options with +20
36
dBm maximum power output for extended range. The wide frequency range can
accommodate RF regulations in different countries. However, it requires different
component value for different frequencies.
Figure 3.12 RF circuit
3.3.4. MicroSD Card Circuitry
The microSD circuit just needs a microSD cardholder. The connections to the
cardholder are shown in Figure 3.13. The microSD card supports SPI communication.
Only R14 and R31 are placed in the microSD card circuitry. In a future version, the
microcontroller universal synchronous asynchronous receiver transmitter (USART) in
37
SPI mode will be used for communication with the microSD card. The microcontroller
supports DMA access to the USART. Using the microcontroller direct memory access
(DMA) capability for writing to the microSD card will decrease power consumption.
Figure 3.13. MicroSD card circuit
3.3.5. Alarm Circuitry
The alarm schematic is shown in Figure 3.14 and consists of an LED (D9) and a
buzzer (SG2) with separate activating lines. Each is activated by the microcontroller
when a monitored parameter is out of the range set by the user.
38
Figure 3.14. Sound and light alarm circuit
3.4. Base-Station Circuitry
Base-station circuitry consists of the following:
1.
Power supply circuitry (PS) for controlling the power supply voltage
2.
Microcontroller circuitry (MCU), that controls all the circuits
3.
Wireless communication circuitry (RF)
4.
Serial communication.
The wireless sensor contains all the base-station sub-circuits, including the serial
communication, which it does not use. Therefore, a wireless sensor is used as a basestation. It requires different software and a serial-to-universal serial bus (USB) converter.
In the project, a serial-to-USB converter is used that also provides 3.3 V to power the
circuit, eliminating the need for a separate power supply. The base-station communicates
with the PC at 115200 bps.
39
CHAPTER 4
4. WIRELESS SENSOR SOFTWARE
This chapter describes the software of the wireless sensor microcontroller. The
software is written in C language, and the CVAVR compiler from HP InfoTech
(Bucharest, Romania) is used.
4.1. Microcontroller Main Routine
After powering up, the wireless sensor configures the ports and the real time clock
(RTC) for 2 s, and then goes into power save mode. The flow chart for wireless sensor
initialization is shown in Figure 4.1. All processes start after the RTC interrupt, which
occur every 2 s if the wireless sensor is in the “Sleep” mode and every 10 ms if it is in
active mode.
Start
Initialization
Sleep
Figure 4.1. Wireless sensor initialization
The processes that the microcontroller performs after each RTC interrupt are
shown in the flow chart Figure 4.2. In the “Sleep” mode, after each RTC interrupt, the
40
REAL TIME CLOCK INTERRUPT
YES
NO
IS T
INTERVAL
>1S?
IS ON
MODE?
NO
YES
GET TEMPERATURE
CHECK IF RX DATA
YES
IS RF DATA?
IS
ACCELEROMETER
ON?
UPDATE SETTINGS
YES
GET ACCELEROMETER
NO
NO
YES
IS
OXIMETER
ON?
GET OXIMETER
NO
IS ALARM
STATUS
CHANGED?
NO
YES
SET ALARM ON\OFF MODE
IS RX
RECEIVED?
YES
IS SD ON?
NO
YES
SD FUNCTION
UPDATE SETTINGS
NO
YES
IS TX
BUFFER
FULL?
NO
NO
IS RX TIME?
NO RX FOR
>18S?
NO
SET SLEEP 10US
YES
SER SLEEP 2S
YES
TRANSMIT\RECEIVE DATA
SLEEP
Figure 4.2. Wireless sensor microcontroller
41
microcontroller checks for an incoming RF signal. If no signal is received from the basestation, the wireless sensor goes to “Sleep” for 2 s to repeat the procedure again. If a
base-station transmission was received, the wireless sensor updates its settings based on
the received information and then checks again the sleep/on setting and proceeds
accordingly.
If the wireless sensor is in the “On” mode, then it acquires the temperature data if
more than 30 s elapsed since the last temperature acquisition. Then, the microcontroller
checks the accelerometer. If the accelerometer is set for continuous data acquisition, then
the accelerometer data are acquired based on the sampling rate set for the accelerometer.
If the accelerometer is set to generate an out-of-range interrupt, then the microcontroller
checks the interrupt, and if an interrupt is registered, then accelerometer data are
acquired.
Then the microcontroller acquires the pulse oximeter data if the oximeter is
activated. The flow chart for pulse oximeter data acquisition is described in section 4.4.4.
After that, the microcontroller checks the light and sound alarms. If any of the
parameters set to be monitored is out of range, the alarm set to be activated is activated. If
either of the alarms was previously activated, and a command to deactivate is received
from the base-station, then the microcontroller disables the alarm.
Next, the microcontroller checks if the acquired data are to be saved on the
microSD card. The microSD card routine and flow chart are described in section 4.5.
After data acquisition, the microcontroller checks if more than 1 s passed since
the last command from the base-station was received, and if so, then regardless of the
42
amount of data in the buffer, the data are sent to the base-station, along with the code
querying the base-station for a command. If the last RF transmission was less than 1 s
ago, then the microcontroller checks if the 64-byte buffer is full, and if it is full, then the
data are transmitted to the base-station.
If a code querying the base-station for a command was sent, then the wireless
sensor enables the wireless receiver and waits for 2 ms for a command from the basestation. If a command was received from the base-station, then the wireless sensor
updates its settings and goes into power-save mode for the remaining time to complete 10
ms. If no command was received from the base-station for more than 20 s, then the
wireless sensor assumes that the base-station is not in the range and goes into “Sleep”
mode.
4.2. Temperature Acquisition
The temperature sensor is accessed via a three-wire interface and does not accept
any incoming data. It is the only sensor that requires more than 12 bits to represent the
data and does not fit the 12 bits data plus four bits for sensor identification scheme used
for the wireless protocol, which is described in section 4.6. The temperature data are
provided in two’s compliment format and consist of twelve bits plus one sign bit. To fit
the 12+4 format, the sensor data could be trimmed to represent only positive temperatures
or to reduce the resolution.
43
The wireless sensor is intended to be placed on human skin, and negative
temperatures are not expected. Therefore, only positive temperatures are measured for
this project. The code snippet for temperature acquisition is
void get_TMP(void)
{
S16 temp_tmp;
TMP_cnt_SR+=RTC_PERf;
if (TMP_cnt_SR<SR_TMP)return;//is not acquisition time
TMP_ON;
((unsigned char *) &temp_tmp)[1]=spic_master_tx_rx(0);
((unsigned char *) &temp_tmp)[0]=spic_master_tx_rx(0);
if(((unsigned char *) &temp_tmp)[1] & 0x80!=0)return;
//negative temp detected
temp_tmp>>=3;
sensor_data[data_amount++]=((unsigned char *)
&temp_tmp)[1]|ID_TMP;
sensor_data[data_amount++]=((unsigned char *) &temp_tmp)[0];
TMP_OFF;
TMP_cnt_SR=0 .
}
4.3. Accelerometer Acquisition
The 12-bit three-axis accelerometer is accessed via SPI communication. It is
programmed for the ±2 g range. The accelerometer can be set to generate an interrupt
when a value lower and/or higher than preset threshold value is measured. The following
options are available from the GUI:
1.
enabling/disabling the accelerometer
2.
continuous operation or interrupt-based monitoring
44
3.
in continuous operation, setting the data acquisition sampling rate
(maximum 100 Hz)
4.
in interrupt-based monitoring, setting the free fall detection, minimum,
and maximum activity out-of-range detection.
The data acquisition sampling rate refers to the frequency the accelerometer is accessed;
the accelerometer itself is set to a 100-Hz sampling rate.
4.4. Pulse Oximeter Acquisition
4.4.1. Pulse Oximeter Software Overview
If the pulse oximeter is enabled, then regardless of the pulse oximeter sample rate
setting, the data are sampled at 100 Hz. The oximeter sample rate that is set by the user
refers to the rate the sampled data are sent to the PC. The display of detected red and IR
signals is useful for positioning the wireless sensor on a patient. For continuous
monitoring of the percent oxygen saturation in blood and heart rate, the sampling rate is
set to a lower rate. Physiologically relevant changes in the percent oxygen saturation in
blood and heart rate do not change abruptly; therefore, a lower sampling rate is desired to
increase data storage and lower power consumption.
The software for the pulse oximeter routine has the following hardware access
and control:
1.
Turning on and off and adjusting the intensity of the red and IR LEDs.
45
2.
Differential DAC with gain to sample the output of the second stage
photodetector amplification circuitry.
3.
DAC voltage output to the second stage non-inverting amplifier input for
controlling the second stage amplifier output offset.
4.
DAC to the output of the first stage amplifier. These data are used for adjusting
the intensity of the IR and red LEDs.
Briefly, the pulse oximeter software turns on the IR LED and applies a voltage
offset to the second stage amplifier, waits for the processes to settle, takes measurements
of the first and second amplifier outputs, turns the IR LED off and removes the offset.
After that, the microcontroller checks if the output from the second amplifier is in the
DAC measurable range. If data are not in range, the microcontroller uses the data from
the first amplifier stage to calculate the new LED intensity for the next measurement.
Then it does the same procedure with the red LED. If the red, IR, or both red and IR data
are out of range, then new LED intensities are calculated. This procedure eliminates
creating a software loop for adjusting the light intensity. It takes up to 10 to 20 s to get
the signal in range when the wireless sensor is placed on a patient or when the
surrounding conditions change for a prolonged time. But, in this way, attempts to adjust
the LED intensities to accommodate out of range signals caused by short duration motion
artifacts are minimized. The signal from the second stage amplifier is filtered and is used
to calculate the heart rate and percent oxygen saturation in the blood. After that, based on
the user setting, the filtered wave data or calculated heartbeats and percent oxygen
46
saturation are sent to the transceiver to be transmitted to the PC or saved on the wireless
sensor microSD card.
4.4.2. Controlling the LEDs and Taking Data Samples
Every 10 ms, if the oximeter is active, the pulse oximeter routine starts with
connecting the IR LED’s anode to a high voltage, by reconfiguring the corresponding
microcontroller control pin from input to a pulled up output. The output voltage from the
DAC is connected to the common LED anode. The decrease in the DAC voltage causes
an increase in LED intensity. The voltage from the second DAC channel is applied to the
second stage non-inverting amplifier input for adjusting the output offset. After that, the
microcontroller is put in “Sleep” mode for 500 µs. The on/off red and IR LED sequences
are shown in Figure 4.3. The voltage rise on the output of the first stage in response to the
LED light is shown in Figure 4.4.
A timer-interrupt wakes up the microcontroller after 500 µs. After this time, all
the processes settle. The LED light and the amplifier output voltages are settled. The
microcontroller samples the outputs of both amplifier stages. The second stage amplifier
is sampled by the ADC configured as a differential input with gain. For the differential
input configuration, the microcontroller has 1x, 2x, 4x, 8x, 16x, 32x, or 64x options for
software selectable gain. In the project, the gain is adjusted based on the maximum wave
swing, with a maximum gain limit of the 8x.
47
Figure 4.3. IR (bottom) and red (top) LEDs on/off sequence
Figure 4.4 First stage amplifier settling time
48
The code snippet for controlling the red LED and sampling both amplifiers is
dacb_write(1,Roffset); //apply v offset to the non-inverting input, second
stage
dacb_write (0,Rintensity);//apply v to LED’s cathode
R_LED_ON;
//apply high voltage to red LED anode
SET_t_out_us (500);
//set time out
idle ();
//set microcontroller in idle
ad_R_data= adca_read1 (0);//sample second stage amplifier
in_level_R= adca_read (3); //sample first stage amplifier
R_LED_OFF;
//turn red LED off
The ac component of the useful signal from the first stage has a swing of 3-10
ADC units. This swing does not affect the measurement for detecting the dc component,
which could be up to 300 times bigger. For example if the measured signal is 3000 ADC
units, with a useful signal swing of 3-10 ADC units, 2990 ADC units can be safely
removed without affecting the useful signal.
Figure 4.5. First (top) and second (bottom) stage amplifier outputs
49
The code snippet for calculating the light intensity for the red LED is
if (in_level_R>top)
{
if (Rintensity<min_light)Rintensity++;
}
else if (in_level_R<btm)
{
if (Rintensity>0)Rintensity--;
}.
The first and second amplifier stage wave outputs are shown in Figure 4.5.
4.4.3. Red and Infrared (IR) Signal Processing
To suppress noise in the signal, including 60-Hz and 120-Hz noise caused by
fluorescent lights and other electrical equipment, the signal is lowpass filtered. The
heartbeat frequencies of interest are considered to be from 30 to 270 beats/min. That is,
from
30 beats
1 min.
1 min.
= 0.5 Hz
60 s
(0.3)
270 beats
1 min.
1 min.
= 4.5 Hz .
60 s
(0.4)
f=
to
f=
Therefore, a 5-Hz second-order Butterworth Infinite Impulse Response (IIR)
digital lowpass filter is used. Filter coefficients were determined by “WinFilter” software,
50
from http://www.winfilter.20m.com/, that generates 16-bit quantized filter coefficients
and also generates the filter code. The signal obtained while the IR LED is on (IR signal)
and the signal obtained while the red LED was on (Red signal) have independent filter
functions. The code snippet for filtering the Red signal (see Figure 4.6) is
int R_lpf2_5hz (int NewSample)
{
static signed long int y[]={2024,2024,2024};
static signed int x[]={2024,2024,2024};
const int ACoef[] = { 10543, 21086, 10543};
const long int BCoef[] = {16384, -25575, 10507 };
const char NCoef=2;
const char DCgain=32;
long int A,B;
U8 n;
//shift the old samples
for(n=NCoef; n>0; n--) {
x[n] = x[n-1];
y[n] = y[n-1];
}
//Calculate the new output
x[0] = NewSample;
y[0] = ACoef[0];
y[0] *= x[0];
for(n=1; n<=NCoef; n++)
{
A=ACoef[n];
A*=x[n];
B= BCoef[n];
B *= y[n];
y[0]+= A - B;
}
y[0] /= BCoef[0];
return (y[0] / DCgain);}.
51
Figure 4.6. Displaying unfiltered red and filtered IR signals.
The figure shows the effect of the highpass filer. The red signal (on RED channel) is
unfiltered. The IR signal (on IRED channel) is filtered by a highpass filter.
The calculation of the percent oxygen saturation in the blood needs the maximum
and minimum values of both signals. The code for determining these values for the red
signal is
if (ad_IR_data > s_max_ir) s_max_ir = ad_IR_data;
if (ad_IR_data < s_min_ir) s_min_ir = ad_IR_data;.
For determining the number of heartbeats per min., the IR signal is passed
through a highpass first-order IIR digital filter and then rectified. The code for the
highpass filter and rectifier is
hpf_data = (hpf9 (ad_IR_data));
if (hpf_data < 0) hpf_data = 0; //rectifier
int hpf9 (int NewSample)
{
static signed long int y[]={2024,2024};;
static signed int x[]={2024,2024};;
const int ACoef[] = { 25391,-25391 };
const long int BCoef[] = { 32768, -18014 };
long int A,B;
x[1] = x[0];
52
y[1] = y[0];
//Calculate the new output
x[0] = NewSample;
y[0] = ACoef[0];
y[0] *= x[0];
A=ACoef[1];
A*=x[1];
B= BCoef[1];
B *= y[1];
y[0]+= A - B;
y[0] /= BCoef[0];
return (y[0]);
}.
For visualizing the shape of the highpass filter, the signal was temporarily redirected to
the temperature channel, and the waveform is shown in Figure 4.7.
Figure 4.7. Pulse detecting signal (on T channel)
4.4.4. Calculating the Heartbeats
The resulting signal, obtained after highpass filtering and shown in Figure 4.7, is
very clean, and it is easy to calculate the heartbeats. The code snippet is
53
time++;//10ms period
if (hpf_data>threshold && detect_beat==0)
{
detect_beat=1;
beats++;
}
else if(hpf_data==0 && detect_beat==1)
{
detect_beat=0;
beat_min.=6000/time; // (1/ (t*10ms)*1000ms/s*60sec/min
time=0;
}.
For the signal depicted in Figure 4.7, the heartbeat calculation is correct for a threshold
level of zero. For the signal depicted in Figure 4.6, the threshold should be higher to
eliminate the signal “bumps” that appears in between heartbeats.
To suppress signal artifacts created by motion, a more complicated algorithm for
detecting noise and calculating the heartbeats has been implemented. The algorithm
analyzes the signal to detect heartbeats, noise, and out-of-range signal. An out-of-range
signal is a signal that exceeds the minimum or maximum allowable signal duration.
The maximum allowable period of a signal is calculated from the heartbeat
frequency in (0.3) and is
TMAX =
1000 ms
= 2000 ms .
0.5 Hz
(0.5)
If no pulse is detected and TMAX is exceeded, then it is possible that the wireless sensor is
removed from the patient or the patient is in a crisis. Therefore, exceeding TMAX is
always a good reason for setting off the alarm.
54
The minimum signal period is calculated from the heartbeat frequency in (0.4)
and is
TMIN =
1000 ms
= 222 ms .
4.5 Hz
(0.6)
When looking for a pulse, the time up to TMIN can be used for determining the threshold
of the noise in the signal. In this project, the threshold for heartbeat detection is the
maximum value of the pulse detect signal during the 200 ms after a heartbeat is detected.
Figure 4.8, depicts a heartbeat corresponding to an 80 beat/min. heart rate. The pulse
detect signal maximum occurs 100 ms after a pulse is detected. If a maximum occurs
after 200 ms, then the pulse is considered to be contaminated with noise and is discarded.
Each valid detected pulse is passed through a second-order averaging filter, and after
detecting 10 heartbeats, the heartbeat rate is averaged.
350
300
IR heart pulse and pulse detect
250
200
150
100
50
0
40
80
120
160
200
240
280
320
360
400
440
480
520
560
600
640
680
720
760
800
840
880
920
960
1000
1040
0
Figure 4.8. IR heart pulse (top) and pulse detect (bottom)
ms
55
4.4.5. Calculating the Percent Oxygen Saturation in Blood
All the calculations are done by the microcontroller. Calculating the ratio of
logarithmic normalized intensities as in (2.1) could be a difficult task for a
microcontroller. The software for oxygen saturation calculation is based on the
relationship of oxygen saturation to the ratio of red to IR [29].
Thus, instead of calculating the ratio of logarithmic normalized intensities, the
ratio of normalized red and IR values obtained by the pulse oximeter are used. That is,
R
VRED
,
VIR
(0.7)
VR
AC RED
DCRED
(0.8)
VIR
ACIR
.
DCIR
(0.9)
where VR and VIR are, respectively,
and
Figure 4.9, [29] shows the ac and dc components of the signal. Then the ratio formula is
R
ACRED / DCRED
.
ACIR / DCIR
(0.10)
56
Figure 4.9. The ac and dc components of the IR signal.
The figure shows that the dc component for the red and IR signals are not equal [29].
This difference in the dc component of the signal is caused mainly by the different light
intensities of the LEDs and by the change in photodiode sensitivity with wavelength.
From Figure 4.9, the dc value is the minimum value of the ac component. As the
minimum and maximum values of each signal are collected, the minimum and maximum
values can be substituted in the ratio formula,
R
ACRED / DCRED
( R max R min) / R min
ACIR / DCIR
( IR max IR min) / IR min
(0.11)
( R max R min) IR min
.
( IR max IR min) R min
(0.12)
R
The signal of the second stage amplifier output, shown in Figure 4.5, decreases with the
increase of the light intensity and is inverted in comparison with the signal shown in
Figure 4.9. The ac portion of the signal remains the same, while the dc portion is
57
calculated as the difference between the high level voltages corresponding to no-light
level and collected maximum values. Therefore, for calculating the dc portion of the
signal, the voltage of the second stage amplifier is measured when the LEDs are off.
Then the formula becomes
R
( R max R min) (Vdark IR max)
.
( IR max IR min) (Vdark R max)
(0.13)
The oxygen saturation is converted from R using the curve shown in Figure 4.10 [29].
Figure 4.10. Percent oxygen saturation versus R/IR ratio.
The figure shows the empirical calibration curve [29]. The 70% to 100% oxygen
saturation range, which is of the interest in this project, can be approximated by a linear
equation.
58
In practice, the
S PO2 a b R
(0.14)
clinical empirical formula for the oxygen saturation is used. The a and b are coefficients
are determined when the pulse oximeter is calibrated and could be determined by
performing the linear fit of the R values of multiple samples using the least squares
method. Because oxygen saturation below 70 % is not of interest, then the a and b
coefficients are 110 and 25 respectively [36]. The software snippet for calculating the
oxygen saturation is
arr = ((long)lpf_dataIR+offset)/(lpf_dataR+offset);// IR/R
R = (((long) (s_max_r-s_min_r))*arr)/ (s_max_ir-s_min_ir);
arr = 110 -25*R;
Sp_O2_mf[rr_array_counter] = OxiMovingAverage(arr);
Sp_O2 = median_filter (Sp_O2_mf); .
The software flow diagram for pulse oximeter acquisition is shown in Figure 4.11.
4.1. MicroSD Card Software
The option to save on the wireless sensor microSD card can be activated by the
GUI and is used for continuous monitoring when the base-station and the PC are out of
RF range. The flow chart of the microSD card function is shown in Figure 4.12.
59
START
SUPPLY OFFSET VOLTAGE TO THE 2ND STAGE AMPLIFIER
TURN INFRARED LED ON
WAIT 500 ms
ADC 1ST AND 2ND STAGES ACQUISITION FOR IR WAVE
TURN INFRARED LED OFF
SUPPLY OFFSET VOLTAGE
TURN RED LED ON
WAIT 500ΜS
ADC 1ST AND 2ND STAGES ACQUISITION FOR R WAVE
TURN RED LED ON
YES
NO
IS THE R AND IR
WAVE IN RANGE?
ADJUST R AND IR LEDS INTENSITY
LPF(IR AND R DATA)
HPF(LPF IR DATA) PEAK DETECTING
NO
IS NOISE
DETECTED?
YES
RESTART PULSE COUNTING
HEART RATE CALULATION
SPO2 CALCULATION
RETURN
Figure 4.11. Pulse oximeter function
60
START
NO
IS 512BYTE
BUFFER FULL?
YES
IS
DIRECTORY
CREATED?
NO
CREATE DIRECTORY
YES
YES
IS FILE
OPENED?
NO
YES
CREATE/OPEN FILE
IS FILE SIZE
< SET SIZE?
NO
CLOSE FILE
IS
DIRECTORY
SIZE < SET
SIZE?
YES
WRITE FILE
NO
CLOSE DIRECTORY
RETURN
Figure 4.12. MicroSD card function
The microSD function creates files of fixed length and directories that contain a
fixed number of files. The maximum possible number of directories on the microSD card
is 256, and each directory contains 256 files with the exception for the last directory.
Created directories contain numeric names from 0 to 255. Created files contain in the file
name the “LOG_” followed by a three-digit number, followed by “.TXT”. If 256
directories have been created, then in the last directory, the microSD function writes
61
beyond 256 files. The name of this and following files in that directory is “LOG”,
followed by a four-digit number. The data written to the card are in binary format and
need a binary-to-ASCII converter, which has been written in Java on a PC. Each saved
file includes a header that is compatible with “LabChart 7”, data acquisition and analysis
software from ADInstruments.
The microSD card internally writes the data in chunks of 512 bytes, which is the
microSD card sector size, regardless of whether the data size passed to microSD card is
smaller. To decrease power consumption, data are kept in the microcontroller in a 512byte buffer. When the buffer is full, the microSD card is accessed, and the data are
written to the microSD card.
4.2. Wireless Communication
The transceiver is programmed to transmit/receive at 200 kbps at 915 MHz. It has
a 64-byte transmit buffer. In active mode, the data are transmitted every time the buffer is
full. If, based on the wireless sensor settings, the buffer fills up in more than 1 s, then the
wireless senor transmits the buffer contents once a second. The wireless sensor queries
the base-station for a command once a second. The first byte in the transmit data string
indicates if the wireless sensor expects communication from the base-station. If there is
no communication with the base-station for 18 s, then the wireless sensor assumes that
62
the base-station is not in RF range and switches to “Sleep” mode. Transmitted data
consist of 12 bits of data from a particular sensor plus a four-bit sensor identifier.
In “Sleep” mode, the transceiver, configured as a receiver, is enabled for 4 ms
every 2 s and waits for incoming communication. If the wireless sensor is not addressed,
then it remains in “Sleep” mode. If the sensor is addressed, then it executes the command
it receives.
63
CHAPTER 5
5. GRAPHICAL USER INTERFACE (GUI)
The GUI has been written in Java. Via the GUI, a user can control, retrieve,
display, and save the data from a specific wireless sensor on a PC. For controlling a
wireless sensor, the sensor needs to be initialized.
5.1. Initializing a Wireless Sensor
Initialization is the process of detecting the wireless sensor, assigning it a specific
name, which can be the patient’s name, and saving that information in the GUI database.
To do this, the user presses the scan button on the GUI. During this process, the GUI
issues wake-up and scan commands. The wireless sensor, which is in “Sleep” mode,
checks for the incoming command every 2 s. When the wireless sensor receives the
wake-up command, it continues to wait for the next command, and when it receives the
scan command, the wireless sensor sends its ID.
5.2. Controlling a Wireless Sensor
A user can change, via the GUI, the wireless sensor mode to “On” or “Sleep”,
activate/deactivate the accelerometer and the pulse oximeter and change the
accelerometer, pulse oximeter, and temperature sampling rates. The sampling interval of
any sensor can be change from 10 ms to 2550 ms, in 10 ms step. The pulse oximeter
sampling interval affects the data transmitted from the wireless sensor. The on-board
64
pulse oximeter sampling interval, if it is activated, is always 10 ms, regardless of the
GUI-specified pulse oximeter transmission sampling interval. The GUI allows grouping
multiple wireless sensors for timed acquisition control. Figure 5.1 shows the GUI timed
acquisition control panel. The number of wireless sensors grouped in timed acquisition
mode depends on the time any wireless sensor can safely not be monitored.
Figure 5.1. GUI timed acquisition control panel
For example, three wireless sensors (see Figure 5.2) were grouped for timed
acquisition. Assume that the “On” and “Sleep” modes were both set to 1 min., which
actually is the minimum time that can be set. Then, the first wireless sensor (John) in the
list will be on for 1 min., then, after 1 min., only the second sensor (Mia) is “On”, and so
on. After the last sensor in the list, all implants are in “Sleep” mode for one min.
65
Figure 5.2. Wireless sensors grouped for timed acquisition
Therefore, each wireless sensor has been on for 1 min., and in “Sleep” mode for 3 min. It
is possible to include in the list the same wireless sensor multiple times, thus allowing
minimizing the “Sleep” time and maximizing the “On” time for a certain wireless sensor.
For example, in the group of sensors in Figure 5.3, the wireless sensor Alexander is “On”
for 1 min. and in “Sleep” for 1 min., while wireless sensor John is “On” for 1 min. and in
“Sleep” for 3 min., for the same 1-min. “On” and 1-min. “Sleep” timed acquisition
settings. This feature is useful when one sensor needs more frequent monitoring. Figure
5.3 shows an example of increasing the time “On” for a specific wireless sensor.
Figure 5.3. Wireless sensors grouped for timed acquisition for different On/Sleep times
66
5.3. Displaying Sensor Data
The GUI has a monitor window that displays the data received from a wireless
sensor. The following functions are available: manual and auto amplitude adjusting to fit
in the display frame and displaying specific data on the entire GUI monitor frame. The
display is useful for positioning the wireless sensor on a person and for real time
monitoring.
5.4. Saving Sensor Data
The GUI allows saving data received from the wireless sensor on the PC. To
enable the function, the user clicks the “Data Logger is Off” button, which turns into
“Data Logger is On”. When the data logger is activated, the text on the button (see Figure
5.4) changes color between red and blue every second. Also the data display background
changes. The data are saved in ASCII format with a header compatible with “LabChart
7” for further viewing and analysis. Figure 5.5 shows saved data plotted in Excel, a
spreadsheet program from Microsoft (Redmond, WA). A new file is created on a daily
basis, and the saved file contains the wireless sensor name and the date.
67
Figure 5.4. Display monitor.
The figure shows the GUI monitor panel with the Data Logger function on. The signals
received from the wireless sensor and displayed on the GUI monitor are saved on the PC.
The pulse detect signal was temporarily redirected to the temperature signal.
68
1900
1800
1700
1600
1500
1
52
103
154
205
256
307
358
409
460
511
562
613
664
715
766
817
868
919
970
1021
1072
1123
1174
1225
1276
1327
1378
1400
Figure 5.5. Plotting saved data in Excel.
The figure depicts the plot of the received wave signal from the wireless receiver (red
pulse oximeter channel)
69
CHAPTER 6
6. BASE STATION INTERFACE SOFTWARE
The base-station is always in receiving mode, except when an activated wireless
sensor requires communication. When the base-station’s wireless sensor receives data, it
generates an interrupt. While waiting for an interrupt, the base-station’s microcontroller
checks the USART for data from the PC. Received PC data are stored in the
microcontroller and then transmitted to the wireless sensor when the sensor queries for
changes in settings.
An activated wireless sensor transmits data when the 64 byte buffer is full or if a
transmission did not occur for 1 s. The transmission rate depends on the user-set sensor
rate. The temperature is sampled once every 30 s. Continuous waveform transmission can
be useful for wireless sensor placement. For a person at rest, heart rate and percent
oxygen saturation in the blood also can be transmitted once every 30 s. If the
accelerometer is set for detecting low and high activity levels, then the RF transmission
rate is low, therefore lowering power consumption.
Once a second, the activated wireless sensor queries the base-station for changes
in settings. The query code is included in the header transmitted with the sensor data.
70
CHAPTER 7
7. TESTING RESULTS
The GUI – base-station – wireless sensor communication link is operational, and
the wireless sensor responds to GUI commands to change “Sleep” and “On” modes. The
GUI monitor panel displays the data received from the wireless sensor. It includes the
data from X, Y, and Z accelerometer axes, the red and IR pulse oximeter wave or number
of heartbeats and percent oxygen saturation in blood, and the temperature.
The GUI temperature monitor displayed 23.7 °C received temperature data, which
corresponded to ambient temperature. The temperature received from the wireless sensor
was verified against a TPI 310 digital thermometer from Test Products International
(Beaverton, OR) and was correct to the first decimal place.
The GUI monitor for each accelerometer axis correctly displayed the changes in
the received waveform in response to sensor motion in the vertical direction and in the
two horizontal directions. The wireless sensor was rotated around three axes. Each
complete rotation caused the data to change over a ± 1 g range. The accelerometer was
tested for a standing, waking, standing, and jumping sequence. The results, saved by the
GUI, and plotted in Excel are shown in Figure 7.1. The accelerometer was positioned
with the Z axis horizontal and in the direction of movement, the Y axis vertical, and the
X axis horizontal and perpendicular to the direction of movement. The number of steps
and number of jumps can be counted from the Z axis data,
71
The detected number of heart beats was checked against a Nellcor Pulse Oximeter
from Covidien (Mansfield, MA). The heartbeat range measured by the Nellcor Pulse
Oximeter was from 74 to 81 beats per min. The number of heartbeats measured by the
wireless sensor was in the same range. When measured simultaneously with both devices,
the difference in detected heartbeat number was from one to three heartbeats. The
wireless sensor was tested on the workbench by placing a finger over the photosensor.
While monitoring the pulse oximeter signals on the GUI monitor, it was observed that the
pulse oximeter was adjusting to the external changes in light, but the signal was disrupted
in response to hand movement, during which the heartbeat rate update occurred more
slowly. The data for oxygen saturation in blood were tested for one point only. The data
were in the same range as the data for the oxygen saturation obtained from the Nellcor
Pulse Oximeter.
The data logging on the microSD card was activated for a few minutes. Then the
microSD card was removed and checked on a PC. As expected, the microSD contained a
new folder “LOG 001”, which contained a file “LOG001.txt”. The binary file was
converted to ASCII using the converter written for this purpose. The data from the
wireless sensor were recorded. The microSD data logger was tested for longer periods of
time with test data.
The PC data logging was enabled for a few minutes, after which it was disabled.
This process was repeated several times. Then, the file was opened. The data, as
expected, were saved in ASCII format. The time the PC data logger was enabled was
properly recorded. The record contained a header compatible with “LabChart 7”.
1
57
113
169
225
281
337
393
449
505
561
617
673
729
785
841
897
953
1009
1065
1121
1177
1233
1289
1345
1401
1457
1513
72
4000
3500
3000
2500
2000
x
1500
y
z
1000
500
0
Figure 7.1. Accelerometer data (three axis)
73
CHAPTER 8
8. SUMMARY, CONCLUSIONS, AND RECOMMENDATIONS
The HSMS was designed for continuous noninvasive monitoring of a person’s
health state. The importance of continuous monitoring is that health problems are
detected when they first occur, before adverse effects develop. Treatment at an early
stage is most effective and less costly than treatment of an advanced health problems.
The system incorporates multiple types of non-invasive sensors that give a
general assessment of a person’s cardio-respiratory status, temperature, and level of
physical activity and do not require a professional health care provider for
implementation. The HSMS is configurable to accommodate a wide variety of
applications. It could be used in emergency rooms and in the hospital for monitoring
post-operative patients to improve patient safety outcomes and reduce health care cost
caused by undetected patient health degradation. In the home, it will reduce unnecessary
routine physician office visits, while increasing health-care quality, as collected data can
be sent to a physician for a daily review. Continuous health monitoring allows elderly to
feel more comfortable when living independently. The system could be used for
monitoring people with specific health problems, such as people with epilepsy. Another
in-home use of the system is for parents monitoring infants for preventing SIDS. For this
or similar applications, where data collection is not necessary, the system can be
configured to activate a sound and/or light alarm when an abnormality, is detected.
74
Wireless communication with a PC allows a person mobility, eliminating the
inconvenience of being strapped to a stationary monitoring system. The wearable
wireless sensor is small enough to be implemented as a patch. The data saved on a PC
can be imported into different acquisition software programs for complex analyses. For
applications where a computer is out of wireless communication range, such as
monitoring the effect of a stressful situation on the health of first responders, the data are
saved on a microSD card for later review.
Activity monitoring in correlation with other vital data the wireless sensor
provides can help in establishing a safe level of physical exercise. Activity monitoring
also can be used for fall detection, balance control evaluation, and determining walking.
The wearable wireless sensor samples temperature at 30-s intervals and provides
temperature data that have a -0.5, +0.8 °C accuracy (in the 36-40 °C range) and 0.125 °C
resolution. The activity is monitored by a three-axis 12-bit accelerometer with maximum
sampling rate of 100 Hz. The activity sensor can be set to detect falls and out-of-range
(below and above a set threshold) activity and can be set for continuous monitoring. The
pulse oximeter sensor provides the number of heartbeats/min and the percent oxygen
saturation in blood in the 30 to 270 beats/min. The sampling interval of any sensor can be
change from 10 ms to 2550 ms, in 10 ms step. The data from the wireless sensor can be
saved and viewed in real time on a PC or can be saved on a microSD if a computer is out
of RF range. The wireless sensor can be set to issue a light and/or sound alarm if an
abnormality (fall, activity out of range, low or no heartbeat) is detected.
75
Each function of the wireless sensor was tested and worked satisfactorily in the
laboratory environment, but the system was not tested for continuous monitoring in a
clinical environment. The wireless sensor has a small size (38 x 30 x10 mm) but is not
enclosed in a case to be applied as a patch. The described benefits of implementing the
system are based on the results of long-term monitoring studies in the literature. An
important feature of the wireless sensor is that it contains multiple noninvasive sensors.
Multiple sensors provide a more complex status of a person health and can help in
identifying measurements errors. For example, motion artifacts can disrupt the pulse
oximeter data, but the activity sensor will show movement. The temperature and activity
sensors performed better than the pulse oximeter sensor. The temperature sensor provided
accurate temperature (-0.5, +0.8 °C accuracy). From accelerometer data, it was easy to
distinguish among standing, walking, and jumping.
For future versions, the RF communication can be changed to Bluetooth wireless
technology, because the new Bluetooth 4.0 is developed for low-power and up to 50
meters communication range. It will be easier to organize sensor networks controlled
remotely from a PC or from mobile devices. For the wireless sensor, a function for
updating the real time clock should be developed. It will be used by the microSD function
for time-stamping saved data. The communication with the microSD card can be changed
from the SPI port to the microcontroller USART port configured for SPI. This change
will allow writing to the microSD card using the microcontroller DMA, which decreases
the load on the microcontroller and saves power. The pulse oximeter sensor should be
tested with more photodetectors (the circuit is designed to accommodate up to three
76
photodetectors but was tested with one only), which could improve light signal reception.
For the light/sound alarms, a method for turning the alarm off needs to be developed for
situations when the sensor is operating out of PC range. The LED for the light alarm
could be changed to a through-hole type for better light visibility.
77
REFERENCES
[1]
C. Blydenburgh, “Increase in Healthcare Demands Due to the Aging Baby
Boomers,” Yahoo! Contributor Network. [Online]. Available:
http://voices.yahoo.com/increase-healthcare-demands-due-aging-baby11832625.html. [Accessed: 15-Nov-2012].
[2]
D. Rodriguez, “Preventing Epilepsy Seizures - Epilepsy Center EverydayHealth.com.” [Online]. Available:
http://www.everydayhealth.com/epilepsy/preventing-epilepsy-seizures.aspx.
[Accessed: 25-Oct-2012].
[3]
A. H. Taenzer, J. B. Pyke, S. P. McGrath, and G. T. Blike, “Impact of Pulse
Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers,”
Anesthesiology, vol. 112, no. 2, pp. 282–287, Feb. 2010.
[4]
Centers for Disease Control and Prevention, “CDC - Sudden Infant Death
Syndrome (SIDS) and Sudden, Unexpected Infant Death (SUID) - Reproductive
Health.” [Online]. Available: http://www.cdc.gov/sids/. [Accessed: 27-Oct-2012].
[5]
Y. Mendelson, R. J. Duckworth, and G. Comtois, “A Wearable Reflectance Pulse
Oximeter for Remote Physiological Monitoring,” in Engineering in Medicine and
Biology Society, 2006. EMBS’06. 28th Annual International Conference of the
IEEE, New York, NY, 2006, pp. 912–915.
[6]
A. Tura, L. Quareni, D. Longo, C. Condoluci, A. van Rijn, and G. Albertini,
“Wireless Home Monitoring and Health Care Activity Management Through the
78
Internet in Patients with Chronic Diseases,” Med Inform Internet Med, vol. 30, no.
4, pp. 241–253, Dec. 2005.
[7]
“Remote & Wireless Patient Monitoring Markets : Kalorama Information.”
[Online]. Available: http://www.kaloramainformation.com/Remote-WirelessPatient-2645944/. [Accessed: 27-Oct-2012].
[8]
K. Robertson, “Intel-GE Device Gets FDA Green Light - Silicon Valley / San
Jose Business Journal.” [Online]. Available:
http://www.bizjournals.com/sanjose/news/2011/03/04/intel-ge-device-gets-fdagreen-light.html. [Accessed: 25-Oct-2012].
[9]
G. Kelly, “Body Temperature Variability (Part 2): Masking Influences of Body
Temperature Variability and Review of Body Temperature Variability in
Disease,” Altern Med Rev, vol. 12, pp. 49–62, 2007.
[10] J. M. S. Pearce, “A Brief History of the Clinical Thermometer,” QJM, vol. 95, pp.
251–252, 2002.
[11] “Answer to: What is the Relationship Between Physical Exercise and Health?,”
Answers.com. [Online]. Available:
http://wiki.answers.com/Q/What_is_the_relationship_between_physical_exercise
_and_health. [Accessed: 15-Nov-2012].
[12] K. Crowe, “Heart Damage Risk from Excess Endurance Training - Health - CBC
News.” [Online]. Available:
http://www.cbc.ca/news/health/story/2012/06/01/marathon-endurance-heart.html.
[Accessed: 15-Nov-2012].
79
[13] P. D. Thompson, B. A. Franklin, G. J. Balady, S. N. Blair, D. Corrado, N. A. M.
Estes, J. E. Fulton, N. F. Gordon, W. L. Haskell, M. S. Link, B. J. Maron, M. A.
Mittleman, A. Pelliccia, N. K. Wenger, S. N. Willich, and F. Costa, “Exercise and
Acute Cardiovascular Events Placing the Risks Into Perspective: A Scientific
Statement From the American Heart Association Council on Nutrition, Physical
Activity, and Metabolism and the Council on Clinical Cardiology,” Circulation,
vol. 115, pp. 2358–2368, 2007.
[14] DAILY MAIL REPORTER, “The Wrong Amount of Sleep ‘Can Age Your Brain
By Up To SEVEN YEARS’,” Mail Online. [Online]. Available:
http://www.dailymail.co.uk/health/article-1382665/The-wrong-sleep-age-brainSEVEN-YEARS.html. [Accessed: 25-Nov-2012].
[15] BeWellBuzz, “Insufficient Sleep Increases Risk of Stroke and Heart Disease,”
BeWellBuzz News. [Online]. Available:
http://www.bewellbuzz.com/newsroom/news/insufficient-sleep-increases-riskstroke-heart-disease/. [Accessed: 25-Nov-2012].
[16] B. F. Macdonald-Smith, “Can Sleep Deprivation be the Cause of Mental
Illness?,” Telegraph.co.uk, 02-Mar-2009.
[17] D. Freeman and J. Freeman, “Can Lack of Sleep Drive You Mad?,” The
Independent. [Online]. Available: http://www.independent.co.uk/life-style/healthand-families/features/can-lack-of-sleep-drive-you-mad-1705954.html. [Accessed:
25-Nov-2012].
80
[18] M. Hoeffel, “Lack of Sleep Caused 250,000 Vehicle Accidents Last Year,”
Examiner.com. [Online]. Available: http://www.examiner.com/article/lack-ofsleep-caused-250-000-vehicle-accidents-last-year. [Accessed: 25-Nov-2012].
[19] M. Lee, J. Kim, K. Kim, I. Lee, S. H. Jee, S. K. Yoo, “Physical Activity
Recognition Using a Single Tri-Axis Accelerometer,” Lecture Notes in
Engineering and Computer Science, vol. 2178, pp. 14–17, 2009.
[20] M. Sollitto, “Automatic Fall Detection: Call for Help Automatically when Seniors
Fall - AgingCare.com,” AgingCare. [Online]. Available:
http://www.agingcare.com/Articles/automatic-fall-detection-calls-for-helpautomatically-143104.htm. [Accessed: 25-Nov-2012].
[21] C.-C. Yang and Y.-L. Hsu, “A Review of Accelerometry-Based Wearable Motion
Detectors for Physical Activity Monitoring,” Sensors, vol. 10, no. 8, pp. 7772–
7788, Aug. 2010.
[22] J H Eichhorn, “Pulse Oximetry Monitoring and Late Postoperative Hypoxemia on
the General Care Floor,” J Clin Monit Comput, vol. 14, pp. 49–55, 1998.
[23] G. Ingram and N. Munro, “The Use (or Otherwise) of Pulse Oximetry in General
Practice,” Br J Gen Pract, vol. 55, pp. 501–502, 2005.
[24] Y. Gang and M. Malik, “Heart Rate Variability Analysis in General Medicine,”
Indian Pacing Electrophysiol J, vol. 3, pp. 34–40, 2003.
[25] J. Nolan, P. D. Batin, R. Andrews, S. J. Lindsay, P. Brooksby, M. Mullen, W.
Baig, A. D. Flapan, A. Cowley, R. J. Prescott, J. M. M. Neilson, and K. A. A.
Fox, “Prospective Study of Heart Rate Variability and Mortality in Chronic Heart
81
Failure Results of the United Kingdom Heart Failure Evaluation and Assessment
of Risk Trial (UK-Heart),” Circulation, vol. 98, pp. 1510–1516, 1998.
[26] D. M. Kallio, K. Suominen, A. M. Bianchi, T. Mäkikallio, T. Haapaniemi, S.
Astafiev, K. A. Sotaniemi, V. V. Myllylä, and U. Tolonen, “Comparison of Heart
Rate Variability Analysis Methods in Patients with Parkinson’s Disease,” Med.
Biol. Eng. Comput., vol. 40, pp. 408–414, 2002.
[27] L. Walsh, B. R. Greene, A. Burns, and C. N. Scanaill, “Ambient Assessment of
Daily Activity and Gait Velocity,” in 2011 5th International Conference on
Pervasive Computing Technologies for Healthcare (PervasiveHealth), 2011, pp.
418 –425.
[28] R. G. Haahr, “Reflectance Pulse Oximetry Sensor for the Electronic Patch.”
M.Sc. Thesis. Technical University of Denmark, Denmark, 2006.
[29] J. T.B. Moyle, Pulse Oximetry, 2nd ed. BMJ Books,Tavistock Square,London,
2002.
[30] A. Jubran, “Pulse Oximetry,” Crit Care, vol. 3, pp. R11–R17, 1999.
[31] T. O’Dwyer, “Pulse Oximetry System Design.” Analog Devices.
[32] J. G. Webster, Design of Pulse Oximeters. Taylor & Francis Group, New York,
NY, 1997.
[33] C. Valdez-Lowe, S. A. Ghareeb, N. T. Artinian, “Pulse Oximetry in Adults,” AJN,
American Journal of Nursing, vol. 109, no. 6, pp. 52–59, Jun. 2009.
[34] Ledtronics, “SML12R3KIR941T-TR Datasheet.” Ledtronics, Mar-2007.
82
[35] Vishay Semiconductors, “VBPW34S Datasheet.” Vishay Semiconductors, Aug2011.
[36] J. E. Scharf, S. Athan, and D. Cain, “Pulse Oximetry Through Spectral Analysis,”
in Biomedical Engineering Conference, 1993, Proceedings of the Twelfth
Southern, 1993, pp. 227 –229.