FAQ'S Please Note: The Tulane University HRPO website is an... Investigator Guidance tab includes General Guidance Documents, Checklists, Tips and
advertisement

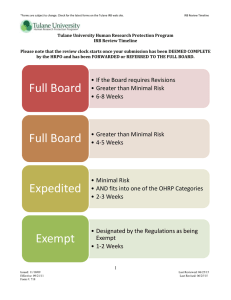
FAQ'S Please Note: The Tulane University HRPO website is an excellent source of information. The Investigator Guidance tab includes General Guidance Documents, Checklists, Tips and Templates, and Specific Guidance Documents. Under General Guidance, there is a very helpful presentation entitled, “10 Steps to IRB Approval”; this presentation has proven to provide assistance as new PIs apply for IRB approval of their human subjects research. Please view our website: http://tulane.edu/asvpr/irb/ You may contact us via email at irbmain@tulane.edu or via phone at 504-988-2665. 1. What is an IRB? An institutional review board (IRB) is a committee that has been formally designated to approve, monitor, and review biomedical and behavioral research involving humans. They conduct a risk-benefit analysis in an attempt to determine whether or not research should be completed. The number one priority of IRBs is to protect human subjects from physical or psychological harm. In the United States, the Food and Drug Administration (FDA) and Department of Health and Human Services (specifically Office for Human Research ProtectionsOHRP) regulations have empowered IRBs to approve, require modifications in planned research prior to approval, or disapprove research. 2. What qualifies as human subjects' research (and thus requires IRB approval)? A. Definition of a Human Subject under the Common Rule, 45 CFR 46 Human Subject means a living individual about whom an investigator (whether professional or student) conducting research obtains (1) data through intervention or interaction with the individual, or (2) identifiable private information. Intervention includes both physical procedures by which data are gathered (for example, venipuncture) and manipulations of the subject or the subject’s environment that are performed for research purposes. Interaction includes communication or interpersonal contact between investigator and subject. Private information includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (for example, a medical record). Citation from the Code of Federal Regulations—Section 102(f) of 45 CFR 46 Note: Private information must be individually identifiable in order for obtaining the information to constitute research involving human subjects (i.e. the identity of the subject is or may readily be ascertained by the investigator, whether an investigator at Tulane or at another institution, or associated with the information). B. Determination of Human Subject Involvement The key questions to answer in determining whether a research activity involves a “human subject” are: 1. Will data ABOUT a living individual be obtained through an intervention or interaction with that individual? 2. Will identifiable private information be obtained about a living individual? If the answer to either question is yes, the research involves “human subjects” and the proposed research proposal must be submitted to the HRPO for the appropriate review and approval prior to initiating the research. C. Additional Aids to Determination of Whether “Human Subjects” are Involved in Research Examples of Research Activities that do not involve “human subjects” The study of individually identifiable private information about dead persons, and no intervention or interaction with living individuals will occur; the study of public information about living persons, and no intervention or interaction with living individuals will occur; and the study of publicly available information files, such as publicly available census files, in which persons are not identifiable (including, but not limited to, the presence in the file of names; social security numbers; codes which the owners of the file or a researcher could reasonably use to identify a living individual; combinations of information from which a person’s identity could easily be determined, such as zip code and birth date) and no intervention or interaction with living individuals will occur Note: The preceding bulleted activities would meet the Federal regulatory definition of “human subject” if anyone, at any point in time, beginning with the commencement of the proposed research, holds information necessary to link a file record to the identity of any living person and the information in that record would be considered private information. D. Definition of Human Subject Decision Chart A decision chart entitled, “Chart 1: Is an Activity Research Involving Human Subjects Covered by 45 CFR 46?” from the Office of Research Protections (OHRP) of the U.S. Department of Health and Human Services (DHHS) may be helpful in determining whether a research proposal involves “human subjects” as defined in HHS Regulations (45 CFR 46 Common Rule). You can find this and other decision charts on the OHRP website: http://www.hhs.gov/ohrp/policy/checklists/decisioncharts.html 3. I need to obtain IRB approval, where do I start? Complete human subjects research training (CITI Training). Before beginning research at Tulane University, all research personnel must complete the CITI Training Program. A Certificate of completion is to be submitted for all research personnel when applying for IRB approval. CITI Training can be completed online at: www.citiprogram.org If the research is Biomedical, all study team members should complete the Biomedical (group 1) Research for Investigators and Key Personnel course. If the research is Social Behavioral, all study team members should complete the Social and Behavioral (group 2) Research for Investigators and Key Personnel course. CITI Training certificates expire four years from the completion date and the completion date is listed on the certificate. Please see the CITI Training Guidance Document on our website for more detailed information regarding completing CITI Training and how to submit the Certificate(s) for review. 4. What do I need to submit? The Tulane University HRPO has created a thorough Initial Submission Checklist for Expedited/Full Board submissions and a Checklist for Exempt submissions. These can be found on our Website by clicking the Investigator Guidance tab, under the heading ‘Checklists’ http://tulane.edu/asvpr/irb/ 5. How do I know if I am applying for Expedited/Full Board review or for Exempt Review? The OHRP has issued categories of research. The Tulane University HRPO has Guidance Documents to assist you in determining if your research may qualify as Exempt or for Expedited review. The research may be referred to the Full Board for a determination if the research involves a greater than minimal risk to subjects or involves a vulnerable population. Please see the Guidance Documents on our website under Investigator Guidance entitled: Types of Review Criteria for Exempt Determination Criteria for Expedited Determination http://tulane.edu/asvpr/irb/ 6. How do I submit an Application for IRB review? All Tulane submissions are made through IRBNet, an electronic submission and study management system. Use of the system requires you to create an account with IRBNet. You will find all our Forms, Checklists, Templates, etc. on the IRBNet system. www.irbnet.org The Tulane University HRPO has constructed an IRBNet Users Guide, which walks users through the submission system step-by-step. This can be found on the HRPO website. In addition, there is a short video of how to put together an initial submission. This helpful video can also be found on the HRPO website. http://tulane.edu/asvpr/irb/ 7. When is my submission going to be processed? The length of time that it takes to review an IRB submission depends significantly on the quality of the application, when it is received by the IRB, and the type of review that it requires. Submissions that are incomplete will not be processed until all documents have been submitted to the IRB. Please see the Review Timeline Table below. For Full Board submissions, there are submission Deadlines; please see our website and click the Deadlines and Meetings link. Keep in mind that incomplete submissions will delay the amount of time it takes to process and review your application. 8. How will I know that I have approval to conduct my research? Once your Application is reviewed by a board members, a Decision Letter will be published within IRBNet. You will receive an email notification that a letter has been published. To locate decision letters: INSTRUCTIONS FOR LOCATING YOUR ACTION/DECISION LETTER Step 1: Login to IRBNet; www.irbnet.org. This will take you to the MY PROJECTS page. Step 2: Click on the Title of the project for which you would like to retrieve the action letter. Step 3: Click on PROJECT HISTORY and then the PACKAGE TYPE of the specific submission (package #). Step 4: Click on PROJECT OVERVIEW and then REVIEW DETAILS. The action letter is located in the BOARD DOCUMENTS section at the bottom of the page. 9. Once IRB Approval has been obtained, do I have to maintain it? Yes, any Amendment to the research (Protocol, Consent Documents, Questionnaires, etc.) are to be reviewed and approved by the IRB prior to implementing. Continuing Reviews are required of all Expedited and Full Board studies, as these studies will expire. Any Protocol Deviations and Unanticipated Problems are to be submitted to the IRB for review. In addition, once all research has been completed, including all analysis of collected study data, a Study Closure is to be submitted. Please see our website under Investigator Guidance, the Specific Guidance Documents contain information on these types of submissions. http://tulane.edu/asvpr/irb/ 10. Can an investigator begin research on human subjects without prior IRB approval? No, all research involving human subjects must obtain final IRB approval prior to beginning any and all research activities (including subject recruitment). Human subjects research conducted without IRB Approval is considered investigator non-compliance. Other Topics: What if I were to forget to submit my application for continuing review? As a courtesy to investigators, the IRB sends investigators study renewal reminder(s) prior to the expiration of the study. It is the responsibility of the Principal Investigator to ensure that the continuing review submission is submitted via IRBNet in sufficient time to be reviewed prior to the study's expiration. You may submit continuing review applications 60 days prior to the expiration date, and you should ensure that a continuing review has been submitted at least 30 days before the expiration date to allow enough time for the IRB review. If an investigator has failed to provide continuing review information to the IRB, or the IRB has not reviewed and approved a research study by the continuing review date, IRB approval expires automatically and all research activity must cease, unless the IRB finds that it is in the best interests of individual subjects to continue participating in the research interventions or interactions. The continuation of research after expiration of IRB approval is a violation of the regulations 45 CFR 46.109(e), and 21 CFR 56.103(a). However, if the investigator is actively pursuing renewal with the IRB and the IRB believes that an over-riding safety concern or ethical issue is involved, the IRB may permit the study to continue for a period of 30 days following the expiration date. If a renewal request is received within 30 days of expiration, the request will be processed as continuing review. After 30 days following expiration, the study is officially closed and cannot be reopened. The IRB will administratively close expired studies and send the principal investigator a closure notification. Do HIPAA regulations apply to data sets containing protected health information (PHI)? Yes. Protected health information is any information that relates to the past, present or future physical or mental health or condition of an individual. HIPAA regulations require researchers to have valid authorization for all uses and disclosures of research-related PHI. A valid authorization must include specific elements: - A description of the PHl being used. - A statement of the purpose of the use of PHI. - A list of those who can use the PHI. - A list of those who can receive the PHI, including the possibility of re-disclosure. - A statement that once PHI is disclosed by the recipient it may no longer be protected by the Privacy Rule. - Information about the expiration of the authorization. - Information about the right to revoke the authorization. - Individual identifiable health information consists of: Names - Social security numbers - Geographic information including street address, city, county, precinct, zip code, and their equivalent geocodes. - Voice and fax telephone numbers - Web universal resources locators ( URL ) and Internet Protocol (IP) address numbers Email addresses - Health plan beneficiary numbers, medical record numbers, or other health pla11 account numbers - Vehicle identifiers and serial numbers, including license plate numbers - Device identifiers and serial numbers - Biometrics identifiers, including finger and voice prints - Full face photographic images and any comparable images - All elements of dates (excluding year) including birth date; admission date, discharge date, date of death Information is considered de-identified if all of the above identifiers have been removed, and there is no reasonable basis for belief that the remaining information could be used to identify a particular person