How everything started… Direct link between brain and computer. 1985: Peter Fromherz,
advertisement

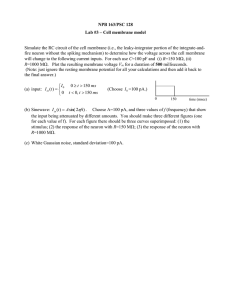
How everything started… Direct link between brain and computer. 1985: Peter Fromherz, Max Planck Institute of Biochemistry How to design a neuron-silicon junction? Neuro-Transistors– JASS 2005 1 Content 1. Neuronal signaling i. ii. iii. 2. Neuron architecture Membrane potential Action potential Neuro-Transistors i. ii. iii. iv. Point-Contact Model Transistor recording Capacitive stimulation Two neurons and a chip Neuro-Transistors– JASS 2005 2 Architecture of a neuron Basic functional units: Input component Dendrites Trigger component Cell body (soma) Long-range conducting component Axon Output component Presynaptic terminals Neuro-Transistors– JASS 2005 3 Signaling Neurons communicate by electrical signaling. Action potential: Brief, invariant and large electrical pulse All-or-none signal Frequency-coded Neuro-Transistors– JASS 2005 4 The cell membrane Double layer of hydrophobic lipid molecules Membrane proteins: 1. voltage-gated ion channels 2. ligand-gated (chemicallycontrolled) ion channels 3. energy consuming ion pumps Control transport of ions through the cell membrane. Neuro-Transistors– JASS 2005 5 Membrane Potential Separation of charges across membrane Membrane potential: VM Vin Vout Potential determined by: Ionic conductances of the cell membrane Distribution of ions across the membrane (mainly potassium K and sodium Na ) Reduction in charge separation: Depolarization Increase in charge separation: Hyperpolarization Neuro-Transistors– JASS 2005 6 Membrane Potential K -ions concentrated Charge separation across the membrane. inside the cell. Chemical force driving them outside down concentration gradient. Fchem Fel Potential difference across the membrane driving K -ions back into the cell. Neuro-Transistors– JASS 2005 7 Membrane Potential Equilibrium: Chemical force = Electrical force Equilibrium potential: Giant squid axon: VK VK k B T K o ln z e K i Nernst Equation 25mV 20 ln 75mV 1 400 Passive process, consumes no energy. Energy is needed to set up initial concentration gradients. Ion pumps: Proteins in cell membrane. Ion transport through hydrolysis of ATP to ADP. Neuro-Transistors– JASS 2005 8 Equilibrium with several ion species: Ion flux = (electrical force + chemical force) x membrane conductance Influence of each ion species by concentration gradient and permeability of membrane Membrane potential described by Goldman-Hodgkin-Katz equation: k B T PK K o PNa Na o PCl Cl i Vm ln e PK K i PNa Na i PCl Cl o Vm GHK Equation usually around -60mV. Neuro-Transistors– JASS 2005 9 Generation of an action potential Membrane potential and ionic conductances computed from the Hodgkin-Huxley model. Neuro-Transistors– JASS 2005 10 Recording electrical signals from neurons: Small glass micropipettes (d < 1µm) filled with concentrated salt solution are inserted into the cell. Connection via an amplifier to an oscilloscope. Neuro-Transistors– JASS 2005 11 Voltage-clamp technique: Difficult to examine ion conductances g K and g Na because of their strong voltage dependence. Holding (clamping) the potential in the cell at a certain value. Opening of voltage-gated ion channels does not affect membrane potential. Neuro-Transistors– JASS 2005 12 Patch-Clamp Technique Allows measurement of currents through single ion channels. Suction Seal between electrode and membrane. Reduction of electronic noise. Neuro-Transistors– JASS 2005 13 Signal Propagation along the Axon Axon can be described as one-dimensional cable. Cell membrane: insulating coat Intracellular fluid: conductive core Conduction velocity depends on: Diameter of axon (Giant squid axon d=1mm) Insulation of axonal membrane Neuro-Transistors– JASS 2005 14 Myelination Myelin: electrically insulating layer around axons. Conduction velocity: Unmyelinated: v = 5 to 10 m Myelinated: up to v = 150 m Nodes of Ranvier Neuro-Transistors– JASS 2005 15 s s Synapses Chemical signal transmission across the synaptic cleft. Action potential Neurotransmitter release Neural plasticity: Regulation of synaptic strength. Learning and memory Neuro-Transistors– JASS 2005 16 Where to go? Today: Trying to understand fundamental principles of neuron-silicon junctions. Physical rationalization of junction to optimize neuron-silicon interfacing Hybrid systems with neuronal networks and microelectronic devices. ? ……….. Today Science-Fiction Neuro-Transistors– JASS 2005 17 Principles of coupling (a) Electrical field across the membrane polarizes the silicon dioxide on the chip. (b) Electrical field across the silicon dioxide polarizes the membrane affecting voltagegated ion-channels. Unfortunately: Proteins from cell membrane keep membrane at certain distance from the chip. (c) Neuronal activity leads to ionic and displacement currents through the membrane. Current spreads along the cleft. Transductive Extracellular Potential (TEP) (d) Voltage transient applied to silicon causes displacement current through oxide. Transductive Extracellular Potential (TEP) Neuro-Transistors– JASS 2005 18 Point-Contact Model Transductive extracellular potential mediates coupling of neuron and silicon. TEP is determined by current balance in junction. Point-Contact Model Capacitance of the Membrane: CM , cM CM AJM Ionic conductances: G i JM i , g JM i GJM AJM Capacitance of the chip: CS , cS CS AJM Conductance of the cleft: G J , g J Neuro-Transistors– JASS 2005 19 GJ AJM Point-Contact Model i I Mionic g JM VM VJ V0i dV dV I Mcap cM M J dt dt i dV dV I Scap cS S J dt dt ohmic I cleft g J VJ VE Kirchhoff‘s law: I cleft I S ohmic cap I Mcap I Mionic dV dV g J VJ VE cS S J cM dt dt dV dV i M J g JM VM VJ V0i dt i dt Neuro-Transistors– JASS 2005 20 Point-Contact Model AFM :Area of the free membrane. AJM :Area of the attached membrane. Current balance in the cell: AJM dVM dVJ i i cM g JM VM VJ V0 dt i dt dV dV i AFM cM M E g FM VM VE V0i dt i dt Neuro-Transistors– JASS 2005 21 Point-Contact Model Remarks: i ionic conductances g JM depend on voltage difference across the membrane (Hodgkin-Huxley-Model). point-contact model assumes that all currents flow through one point in the membrane. Parameters g J , cM , cS represent average values. point-contact model is a simplification of an area-contact model where VJ x, y depends on the position x, y in the junction. Efficient recording and stimulation: small distance d J high specific resistance J large radius a J small g J high ionic conductances high capacitance of the chip Neuro-Transistors– JASS 2005 22 Transistor recording Stimulation voltage Electrolyte-source-voltage VES VDS Source-drain-voltage Source-drain current I D is controlled by gate-source voltage VGS VJ VS Resulting current is changed to a voltage, amplified and watched on an osciloscope. Calibration measurement without cell to determine voltage-current characteristic I D VGS Neuro-Transistors– JASS 2005 23 Ac-stimulation with transistor recording: Leech neuron on FET contacted with patch-pipette in whole cell configuration. Ac-voltage VM t is amplified. Response VJ t recorded with transistor. VJ Plot of transfer spectrum : VM Two different types of spectra observed: A-type: - small amplitude at low frequencies. - increase of phase around 10Hz. - increase of amplitude above 1000Hz. B-type: - high amplitude at low frequencies. - just minor change in phase. - further increase of amplitude above 1000Hz. Neuro-Transistors– JASS 2005 24 Interpretation using the point-contact-model: Insert intracellular stimulation VM t V M e and extracellular response it i VJ t V J eit in g J VJ VE cS dVS dVJ cM dVM dVJ g JM VM VJ V0i dt with VE 0 dVS 0 dt and i g JM dt dt i g JM No ion channels, just leak conductance. dV dV VJ g J g JM J cS cM VM g JM cM M dt dt VJ Low frequency limit: VM dt 0 VJ g JM icM V M g J g JM i cS cM g JM g J g JM A-type: Small amplitude at low frequencies low membrane conductance g JM B-type: Enhanced amplitude at low frequencies larger membrane conductance Further increase at high frequencies large conductance g J Neuro-Transistors– JASS 2005 25 Ac-stimulation with transistor recording: With the values cM 5 F cm 2 and cS 0.3 F cm 2 which are known, data fitting gives us: 2 g J 217 mS cm 2 A-type: g JM 0.36 mS cm 2 B-type: g JM 38.5 mS cm g J 40.8 mS cm 2 Crucial difference: leak conductance of the attached membrane differs by two orders of magnitude. A-type = Capacitive junction B-type = Ohmic junction Neuro-Transistors– JASS 2005 26 Transistor recording of neuronal activity: Small signal approximation: VJ VM V0i , dVJ dVM Small extracellular potential dV dV cS S J 0 dt dt No capacitive current to the chip g J VJ cM 1 M cM dVM i g JM VM V0i dt i dVM i i g FM M g JM VM V0i 1 M jINJ dt i M AFM AJM Neuro-Transistors– JASS 2005 27 Transistor recording of neuronal activity: A-, B- and C-type response: No voltage-gated ion channels in the membrane: g J VJ g JM VM V0 cM Negligible leak conductance dVM dt TEP proportional to first derivative A-type junction Dominating ohmic leak conductance TEP reflects intracellular waveform B-type junction Insert cM dVM 1 i i g FM M g JM VM V0i jINJ 1 M i dt in g J VJ g JMi VM V0i cM i dVM dt Neuro-Transistors– JASS 2005 28 Transistor recording of neuronal activity: g J VJ g i i JM 1 i i VM V 1 g FM VM V0i jINJ M g JM i M g J VJ i 0 1 i i g JM g FM VM V0i jINJ 1 M i TEP of an action potential relies on inhomogeneity of the membrane. Wide spectrum of waveforms VJ t depending on distribution of voltage-gated ion channels. Details must be treated by numerical simulation. Neuro-Transistors– JASS 2005 29 Transistor records of a leech neuron Two positions of the neuron: (a) Cell body right on the transistor. (b) Axon stump on transistor array. Action potential elicited by current injection with a micropipette. Intracellular potential measured with pipette, extracellular potential with transistor. Neuro-Transistors– JASS 2005 30 Transistor records of a leech neuron Three types of records: A: first derivative of waveform capacitive junction B: waveform itself ohmic junction C: numerical simulation: accumulation of K and Na -channels in attached membrane. Depletion of ion-channels in cell body. High density of ion-channels in axon Neuro-Transistors– JASS 2005 31 Transistor records of neurons from rat hippocampus Action potentials are elicited by current injection. Records with signal averaging of transistor signals. Observation: Two positive transients in VJ t , one in rising phase of AP and one in falling phase. Interpretation: Positive peak in rising phase is related with sodium current. Na With VM V0 0 Na Na g JM g FM 0 Sodium inward current through free membrane gives rise to capacitive outward current through attached membrane. Positive peak in falling phase is related with outward potassium current through attached membrane. K K g JM g FM 0 VM V0K 0 Neuro-Transistors– JASS 2005 32 Capacitive stimulation of neuronal activity Changing voltage VS t applied to stimulation spot Capacitive current through insulating oxide Current along the cleft Transductive extracellular potential TEP Voltage-gated ion channels in the membrane may open Action potential VM t may arise. A-type stimulation: Voltage step VS t VS t Exponential response of membrane potential. 0 VM t V e 0 M t J with very short time constant J 3s for mammalian neurons. Neuro-Transistors– JASS 2005 33 Capacitive stimulation of neuronal activity B-type stimulation: Voltage step VS t VS t 0 Exponential response due to capacitive effects. VM Vrest V e 0 M t Also stationary change of intracellular potential. J Vstat C-type stimulation: VM t depends on channel sorting. Must be treated with numerical simulation. Step stimulation of a leech neuron: Voltage step with VS = 4.8, 4.9, 5.0V 0 Stimulation below threshold cannot elicit an action potential (4.8V). Neuro-Transistors– JASS 2005 34 Burst stimulation of snail neuron: Excitation is achieved only when a burst of voltage pulses is applied. After each pulse VM t responds with short capacitive transients at rising and falling edge of VS t After the third pulse the intracellular potential rises so that an action potential is elicited. Neuro-Transistors– JASS 2005 35 Circuits with two neurons on a chip Neuronal activity VM t Probing VJ t with FET Action potential Signal processing Capacitive stimulation (i) Transformation of and amplification. (ii) Identification of an action potential with a threshold device. (iii) Delay line (iv) Generation of a train of voltage pulses. (v) Suppression of crosstalk from stimulator to transistor by refractory unit. Neuro-Transistors– JASS 2005 36 Circuits with two neurons on a chip Connection between a spontaneously firing neuron A along the chip to a separate neuron B. After each action potential in neuron A a burst of voltage pulses is generated and applied to neuron B. Neuron B fires in correlation to neuron A. Neuro-Transistors– JASS 2005 37 Signaling chip-neuron-neuron-chip Action potential Synaptic connection Neuronal activity VM t Probing VJ t with FET Capacitive stimulation Problem: Growing neurites exert strong forces on the cell bodies of neurons. They pull them of their contacts. Neuro-Transistors– JASS 2005 38 Mechanical fixation of the cell bodies Picket fences made out of polyimid (plastic) around each contact. Two neurons from an immobilized network of snail neurons. Stimulation with burst of seven voltage pulses. Third action potential in neuron 1 leads to a postsynaptic excitation in neuron 2. Perturbations in transistor signal due to capacitive coupling with stimulator. Neuro-Transistors– JASS 2005 39 Towards defined neuronal nets Systematic experiments on network dynamics require: (i) Noninvasive, long term supervision and stimulation of neurons (ii) Fabrication of neuronal nets with defined topology of synaptic connections. Control of neuronal outgrowth: 1.) Chemical guidance: Motion of neuronal outgrowth is guided by chemical patterns. Linear patterns of extracellular matrix proteins are able to guide neuronal outgrowth and let them form synapses. Neuro-Transistors– JASS 2005 40 Towards defined neuronal nets 2.) Topographical guidance Grown neurites are immobilized by microscopic grooves. Cell bodies are placed in into the pits of a polymer structure. Neurites grow along the grooves and split at bifurcations. Problem: Neuritic tree is not uniquely defined by the guiding pattern. Alternative: Disordered growth of neuronal nets on closely packed transistor arrays. Neuro-Transistors– JASS 2005 41 Transistor arrays 12 neurons cultured on an array of 128x128 transistors ( 1mm 2). Neurons I, II and III are connected by synapses. Burst of action potentials elicited at Neuron I with a micropipette. Neuro-Transistors– JASS 2005 42 Alternative materials Drawbacks of silicon: (i) Electrochemical instability of silicon dioxide Long-term shift of electrical properties of the FETs. (ii) High noise-level of Si-based devices. Difficult to observe small signals from neurons. Realization of EOFETs with AlGaN/GaN heterostructure FETs. These materials are stable under physiological conditions. Much higher signal-to-noise ratio than Si-based devices. Neuro-Transistors– JASS 2005 43 Alternative materials Cardiac myocyte cells cultivated on surface of a AlGaN/GaN array 50m Neuro-Transistors– JASS 2005 44 Summary and Outlook Basic principles of neuron-silicon junctions are fairly understood. Properties of the cleft Transistor recording Capacitive stimulation Optimization of neuron-silicon contact: Larger capacity of stimulation contacts Lower noise of transistors Deeper understanding of electrical properties of the cell membrane Neuronal networks: Small defined networks of neurons with learning synapses Large networks of neurons on closely packed transistor arrays Neuro-Transistors– JASS 2005 45 The End Thank You for your Attention! Neuro-Transistors– JASS 2005 46 FLIC Microscopy Fluorescence interference contrast microscopy Membrane labelled with fluorescent dye molecules. Excitation and fluorescence of the dye depend on the distance between membrane and silicon. Membrane and silicon dioxide are not in close contact. Neuro-Transistors– JASS 2005 47