Document 15937261

The following kinetic equations can therefore be used describe growth under oxygen limited conditions.
dX dt
K o m
C o
C o
X dC o dt
1
Y o
K
o m
C o
C o
X
Where Co is the concentration of oxygen, µm is the maximum specific growth rate, Yo is the biomass yield from oxygen and
K o is the Monod constant for oxygen uptake.
1
Thus the oxygen uptake rate (OUR) can be described the equation:
OUR
1
Y o
K
o m
C o
C o
X
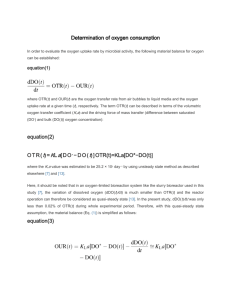
The dissolved oxygen concentration in a reactor is thus determined by the balance between the oxygen transfer rate (OTR) and oxygen uptake rate (OUR).
dC o dt dC o
OTR
OUR dt
k
L a
C
* o
C o
Y o
1
2
K
o m
C o
C o
X
Steady state analysis
The movement of oxygen from a bubble through the bulk liquid to a cell, can be thought of as a continuous process.
Oxygen enters the bulk liquid and is removed by the cells.
3
The dissolved oxygen concentration in the bulk liquid will quickly reach a steady state and therefore dC o dt
0 and the oxygen transfer rate will equal the oxygen uptake rate
OTR = OUR k
L a
C o
*
C o
Y o
1
4
K
o m
C o
C o
X
Critical Oxygen Concentration
•
• C o,cr is the concentration of dissolved oxygen below which a culture is oxygen limited.
Then µ = µ , the cells are not oxygen limited.
5
Integrating the oxygen uptake and oxygen transfer equations
The steady state relationship between oxygen uptake and oxygen transfer:
OTR = OUR k
L a
C o
*
C o
Y o
1
K o m
C o
C o
X
6
level of mixing
7
Medium viscosity
8
Temperature
9
Interfacial area and oxygen transfer
潘威仁 連耘愷
10