Quiz Name_______________________________ 1. (2 points) True or False.
advertisement
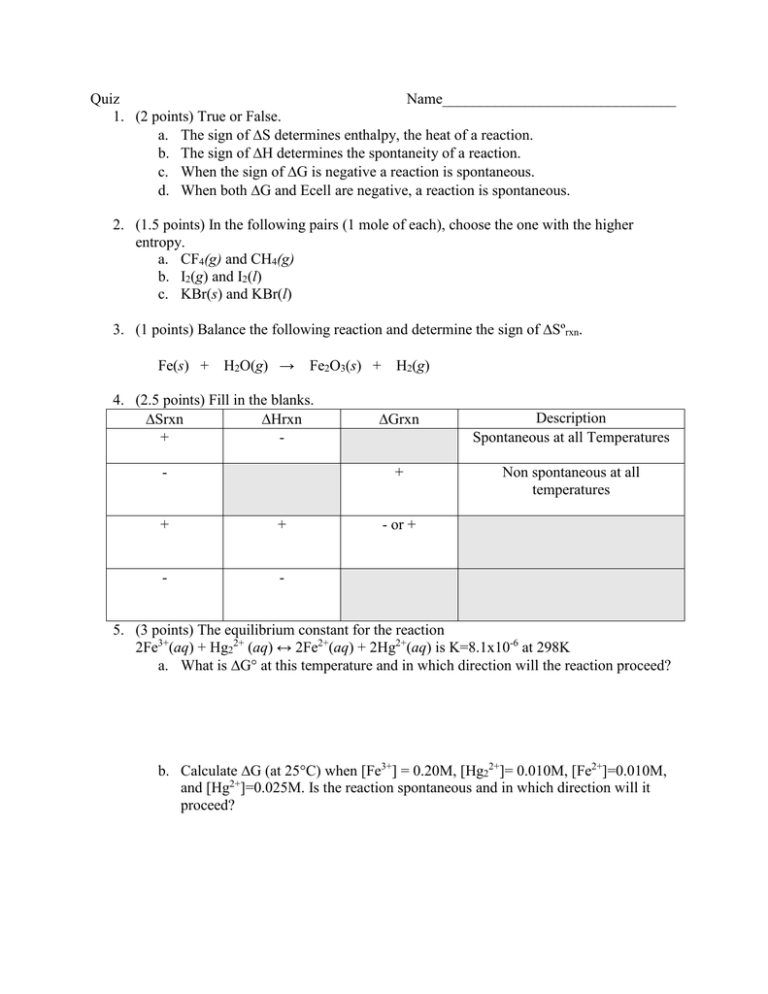
Quiz Name_______________________________ 1. (2 points) True or False. a. The sign of S determines enthalpy, the heat of a reaction. b. The sign of H determines the spontaneity of a reaction. c. When the sign of G is negative a reaction is spontaneous. d. When both G and Ecell are negative, a reaction is spontaneous. 2. (1.5 points) In the following pairs (1 mole of each), choose the one with the higher entropy. a. CF4(g) and CH4(g) b. I2(g) and I2(l) c. KBr(s) and KBr(l) 3. (1 points) Balance the following reaction and determine the sign of Sºrxn. Fe(s) + H2O(g) → Fe2O3(s) + H2(g) 4. (2.5 points) Fill in the blanks. Srxn Hrxn + - + + - - Grxn Description Spontaneous at all Temperatures + Non spontaneous at all temperatures - or + 5. (3 points) The equilibrium constant for the reaction 2Fe3+(aq) + Hg22+ (aq) ↔ 2Fe2+(aq) + 2Hg2+(aq) is K=8.1x10-6 at 298K a. What is G° at this temperature and in which direction will the reaction proceed? b. Calculate G (at 25C) when [Fe3+] = 0.20M, [Hg22+]= 0.010M, [Fe2+]=0.010M, and [Hg2+]=0.025M. Is the reaction spontaneous and in which direction will it proceed?