Chem 121.09 Spring 2012 Exam III Name
advertisement
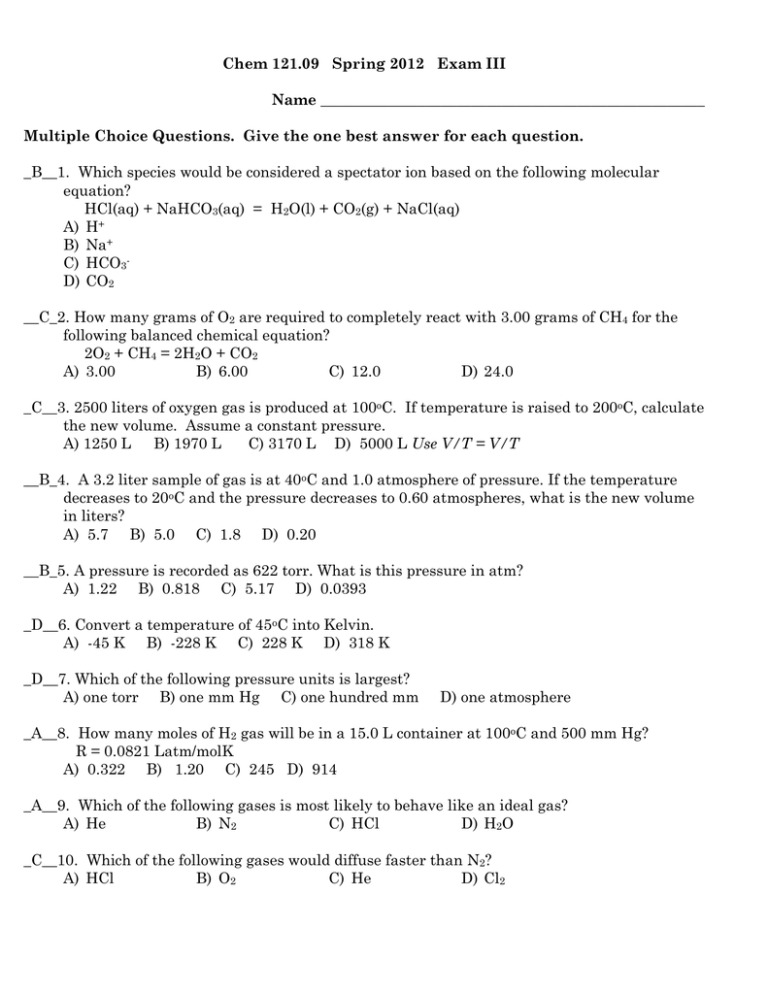
Chem 121.09 Spring 2012 Exam III Name ___________________________________________________ Multiple Choice Questions. Give the one best answer for each question. _B__1. Which species would be considered a spectator ion based on the following molecular equation? HCl(aq) + NaHCO3(aq) = H2O(l) + CO2(g) + NaCl(aq) A) H+ B) Na+ C) HCO3D) CO2 __C_2. How many grams of O2 are required to completely react with 3.00 grams of CH4 for the following balanced chemical equation? 2O2 + CH4 = 2H2O + CO2 A) 3.00 B) 6.00 C) 12.0 D) 24.0 _C__3. 2500 liters of oxygen gas is produced at 100oC. If temperature is raised to 200oC, calculate the new volume. Assume a constant pressure. A) 1250 L B) 1970 L C) 3170 L D) 5000 L Use V/T = V/T __B_4. A 3.2 liter sample of gas is at 40oC and 1.0 atmosphere of pressure. If the temperature decreases to 20oC and the pressure decreases to 0.60 atmospheres, what is the new volume in liters? A) 5.7 B) 5.0 C) 1.8 D) 0.20 __B_5. A pressure is recorded as 622 torr. What is this pressure in atm? A) 1.22 B) 0.818 C) 5.17 D) 0.0393 _D__6. Convert a temperature of 45oC into Kelvin. A) -45 K B) -228 K C) 228 K D) 318 K _D__7. Which of the following pressure units is largest? A) one torr B) one mm Hg C) one hundred mm D) one atmosphere _A__8. How many moles of H2 gas will be in a 15.0 L container at 100oC and 500 mm Hg? R = 0.0821 Latm/molK A) 0.322 B) 1.20 C) 245 D) 914 _A__9. Which of the following gases is most likely to behave like an ideal gas? A) He B) N2 C) HCl D) H2O _C__10. Which of the following gases would diffuse faster than N2? A) HCl B) O2 C) He D) Cl2 _B__11. If H2O(s) → H2O(l) is endothermic and H2O(l) → H2O(g) is endothermic then H2O(g) → H2O(s) A) is endothermic B) is exothermic C) could be either exo or endothermic D) is equal to the sum of the first two reactions. _B__12. The temperature of a liquid is decreased. What happens to the vapor pressure of the liquid as a result? A) it increases B) it decreases C) it is not changed D) it increases or decreases depending on how large a temperature change occurs _D__13. Which of the following influence the vapor pressure of liquids? A) polarity of liquid molecules B) mass of liquid molecules C) temperature of liquid D) more than one response is correct __B_14. Which of the following is not a standard condition (STP) for gas measurements? A) 0OC B) 100 oC C) 273 K D) 760 torr _C__15. In a chemical reaction, it is possible to produce 40.0 g of product but only 35.0 g was produced. What was the percent yield for this reaction? A) 5.00 % B) 12.5 % C) 87.5 % D) 114 % __C_16. An unknown compound is soluble in carbon tetrachloride, a non-polar solvent, but insoluble in water. What can be inferred about the nature of the unknown compound? A) It is ionic. B) It is polar. C) It is nonpolar. D) Nothing can be inferreD) _B__17. When solid ammonium sulfate is dissolved in water, the solution becomes cold. The solution process is A) exothermic B) endothermic C) neither exo nor endothermic D) can't be classified _B__18. The boiling point of a liquid is the temperature at which the vapor pressure of a solution is equal to A) the intermolecular forces B) atmospheric pressure C) 760 Torr D) osmotic pressure _A__19. Evaporation is opposite to A) condensation B) freezing C) melting D) sublimation _C__20. The minor component in a solution is the A) dispersing media B) mixture C) solute D) solvent _B__21. Which of the following substances would most likely decrease in solubility with increasing temperature? A) C12H24O12(sugar) B) CO2 C) MgSO4 D) NaCl _D__22. A solution is made by combining 8.00 g of NaCl and 200 mL of water (density of water = 1.00 g/mL). What is the concentration in % w/w? A) 26.0 B) 4.00 C) 0.0400 D) 3.85 _C__23. How many mL of 12.00 M HCl are needed to prepare 200 mL of 0.200 M HCl solution? A) 0.012 mL B) 0.120 C) 3.33 D) 48.0 __D_24. Two solutions with concentrations of 0.8% sugar and 8% sugar are separated by a semipermeable membrane. During osmosis, there is a net flow of A) sugar molecules from the concentrated to the dilute solution B) sugar molecules from the dilute to the concentrated solution C) water molecules from the concentrated to the dilute solution D) water molecules from the dilute to the concentrated solution _B__25) In comparing a 0.25 molar aqueous NaCl solution to a 0.25 molar aqueous CaCl 2 solution, A) the NaCl solution has the higher boiling point and the lower freezing point. B) the CaCl2 solution has the higher boiling point and the lower freezing point. C) the NaCl solution has the higher boiling point and the CaCl2 solution has the lower freezing point. D) the CaCl2 solution has the higher boiling point and the NaCl solution has the lower freezing point. E) both solutions have the same boiling point and the same freezing point since they have the same molar concentrations. __A_26. Which of the following is not considered a colligative property? A) solubility B) boiling point C) osmotic pressure D) freezing point _C__27. The term hydrophobic matches up in meaning most closely with the term A) hydrophilic B) ionic C) non-polar D) polar _A__28. Which of the following would be considered a strong electrolyte? A) HCl (acid) B) C12H24O12(sugar) C) CaCO3(insoluble) D) HC2H3O2 (vinegar) 29. Na2CO3 solution and CaCl2 solution react together to form insoluble CaCO3 and NaCl solution. Write 3 balanced chemical equations to represent this reaction – a “molecular”, a total ionic and a net ionic. Include the descriptors of (aq), (s), (g), etc as appropriate. Na2CO3(aq) + CaCl2(aq) = CaCO3(s) + 2NaCl(aq) 2Na+(aq) + CO32-(aq) + Ca2+(aq) + 2Cl-(aq) = CaCO3(s) + 2Na+(aq) + 2Cl-(aq) CO32-(aq) + Ca2+(aq) = CaCO3(s) 30. Match each reaction below as A) combination/synthesis B) decomposition C) single displacement D) double displacement E) redox F) non-redox. Each equation will have two correct answers. _AE________Mg(s) + Cl2(g) = MgCl2(s) _DF________BaCl2(aq) + Na2SO4(aq) = BaSO4(s) + 2NaCl(aq) _BF________H2CO3(aq) = H2O(l) + CO2(g) _CE________CuSO4(aq) + Zn(s) = ZnSO4(aq) + Cu(s) 31. Match each condition with a physical state. Mark ALL correct answers. There can be more than one correct answer. __B______great distance between particles A) solids __A______generally have the highest density B) gases __BC____take the shape of their container C) liquids ___C_____particles in contact but not fixed in position ___A_____strongest attractive forces exist 32. Calculate the number of grams of NaCl required to make 500 mL of a 0.20 molar solution? Also determine the concentration of this solution expressed in units of %(W/V). Show your work for full credit. ? NaCl = 0.500 L NaCl X 0.20 mol NaCl/1 L NaCl X 58.5 g NaCl/1 mol NaCl = 5.85 g NaCl 5.85 g NaCl/500 mL solution X100 = 1.17%(W/V) NaCl