Sample Exam 2 (Chapter 5 -6)
advertisement
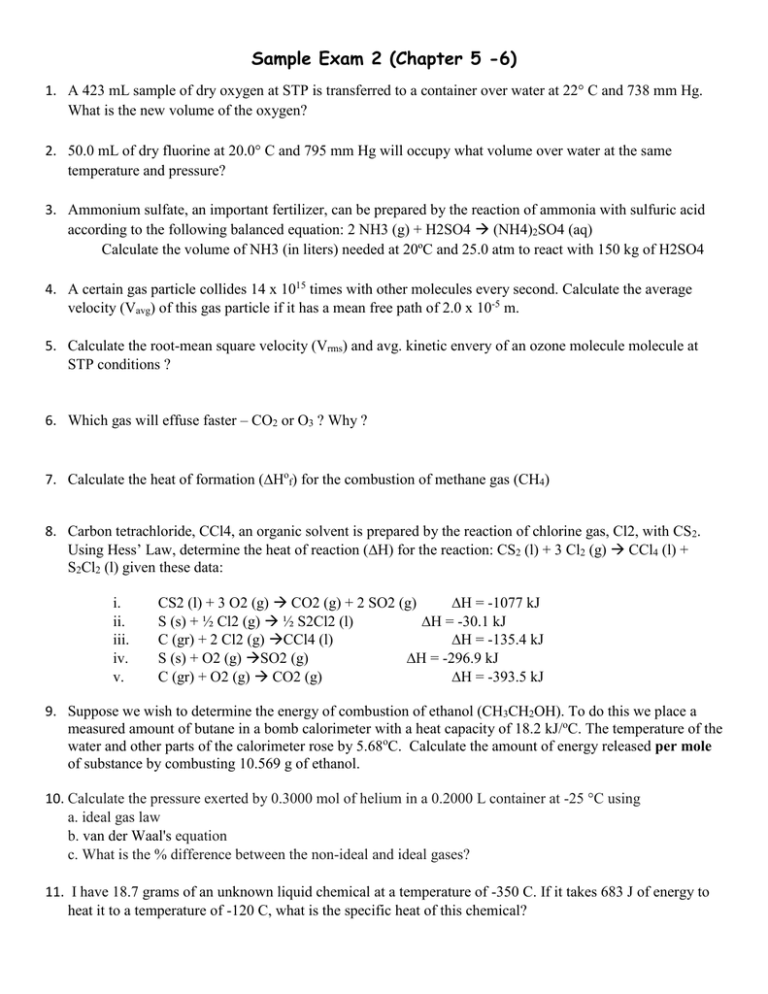
Sample Exam 2 (Chapter 5 -6) 1. A 423 mL sample of dry oxygen at STP is transferred to a container over water at 22° C and 738 mm Hg. What is the new volume of the oxygen? 2. 50.0 mL of dry fluorine at 20.0° C and 795 mm Hg will occupy what volume over water at the same temperature and pressure? 3. Ammonium sulfate, an important fertilizer, can be prepared by the reaction of ammonia with sulfuric acid according to the following balanced equation: 2 NH3 (g) + H2SO4 (NH4)2SO4 (aq) Calculate the volume of NH3 (in liters) needed at 20ºC and 25.0 atm to react with 150 kg of H2SO4 4. A certain gas particle collides 14 x 1015 times with other molecules every second. Calculate the average velocity (Vavg) of this gas particle if it has a mean free path of 2.0 x 10-5 m. 5. Calculate the root-mean square velocity (Vrms) and avg. kinetic envery of an ozone molecule molecule at STP conditions ? 6. Which gas will effuse faster – CO2 or O3 ? Why ? 7. Calculate the heat of formation (Hof) for the combustion of methane gas (CH4) 8. Carbon tetrachloride, CCl4, an organic solvent is prepared by the reaction of chlorine gas, Cl2, with CS2. Using Hess’ Law, determine the heat of reaction (H) for the reaction: CS2 (l) + 3 Cl2 (g) CCl4 (l) + S2Cl2 (l) given these data: i. ii. iii. iv. v. CS2 (l) + 3 O2 (g) CO2 (g) + 2 SO2 (g) H = -1077 kJ S (s) + ½ Cl2 (g) ½ S2Cl2 (l) H = -30.1 kJ C (gr) + 2 Cl2 (g) CCl4 (l) H = -135.4 kJ S (s) + O2 (g) SO2 (g) H = -296.9 kJ C (gr) + O2 (g) CO2 (g) H = -393.5 kJ 9. Suppose we wish to determine the energy of combustion of ethanol (CH3CH2OH). To do this we place a measured amount of butane in a bomb calorimeter with a heat capacity of 18.2 kJ/oC. The temperature of the water and other parts of the calorimeter rose by 5.68oC. Calculate the amount of energy released per mole of substance by combusting 10.569 g of ethanol. 10. Calculate the pressure exerted by 0.3000 mol of helium in a 0.2000 L container at -25 °C using a. ideal gas law b. van der Waal's equation c. What is the % difference between the non-ideal and ideal gases? 11. I have 18.7 grams of an unknown liquid chemical at a temperature of -350 C. If it takes 683 J of energy to heat it to a temperature of -120 C, what is the specific heat of this chemical?






