Essential Chemistry for Biology: Elements, Bonding, Water & pH
advertisement

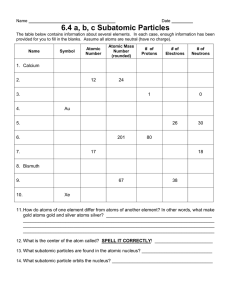
Essential Chemistry for Biology CHAPTER 2 • Elements and the Periodic Table • Atoms and Subatomic Particles • Atomic Number, Mass, and Isotopes of Elements • Chemical Bonding and Molecules • Chemical Reactions • Properties of Water • pH and Acid/Base Balance C - Carbon Periodic Table of the Elements He - Helium Fe - Iron (from ferrum) Al Aluminum Au - Gold (from aurum) Cu - copper (from cuprum) Elements are fundamental categories of matter that cannot be broken down into other types of matter. The Elements Found in Living Things Life is made mostly of “CHON” Figure 2.3 Essential Chemistry for Biology CHAPTER 2 • Elements and the Periodic Table • Atoms and Subatomic Particles • Atomic Number, Mass, and Isotopes of Elements • Chemical Bonding and Molecules • Chemical Reactions • Properties of Water • pH and Acid/Base Balance Every Element (Category) is Composed of Atoms The element gold (Au) Atoms Are the Apples in the Bins Called Elements • Atoms (apples) are the individual items belonging to a specific element (variety) in the Periodic Table. • Atoms belonging to an element are very similar but not identical (they have slightly different masses) Three atoms (apples) from the variety (element) Golden Delicious 24.2 g Periodic Table of Apple Varieties 29.6 g 25.9 g Atoms Are Composed of Three Types of Particles Atoms of an Element Always Have a Unique Number of Protons Boron Carbon Nitrogen Oxygen Fluorine Neon The number of electrons = number of protons in an electrically balanced atom Different Orbits or Shells Have Different Capacities For Electrons Figure 2.7 Essential Chemistry for Biology CHAPTER 2 • Elements and the Periodic Table • Atoms and Subatomic Particles • Atomic Number, Mass, and Isotopes of Elements • Chemical Bonding and Molecules • Chemical Reactions • Properties of Water • pH and Acid/Base Balance A Closer Look at the Numbers For Each Element Numbers That Define Elemental Size and Behavior Elements are organized by: • Size and numbers of parts • Chemical behavior 12.011 Heavier elements are to the right and downwards in rows Elements with similar behavior are in the same column Electron Arrangement and the Chemical Properties of Atoms Atoms of elements with the same number of outer shell electrons have similar chemical behavior. Both nitrogen at bismuth form 3 bonds with neighboring atoms Isotopes are alternate atomic forms for an element. Three “isotopes” of Golden Delicious 24.2 g 29.6 g 25.9 g Mass number is the average mass of the different isotopes found in nature. Radioactive Isotopes can be used to form images Positron Emission Tomography (PET) scan looks for where radioactive fluorine-sugar is being used in the brain (blue areas) Essential Chemistry for Biology CHAPTER 2 • Elements and the Periodic Table • Atoms and Subatomic Particles • Atomic Number, Mass, and Isotopes of Elements • Chemical Bonding and Molecules • Chemical Reactions • Properties of Water • pH and Acid/Base Balance Chemical Bonding and Molecules QuickTime™ and a decompressor are needed to see this picture. QuickTime™ and a decompressor are needed to see this picture. NaCl (sodium chloride) QuickTime™ and a decompressor are needed to see this picture. H 2O Three types of chemical bonds • Ionic bonds • Covalent bonds • Hydrogen bonds Ionic Bonds Are Formed Between Two or More Electrically Unbalanced Atoms (Ions) Covalent Bonds Are Sharing Arrangements Between Two Atoms When A Molecule Becomes Polar Hydrogen Bonds: Bonds Between Polar Molecules Essential Chemistry for Biology CHAPTER 2 • Elements and the Periodic Table • Atoms and Subatomic Particles • Atomic Number, Mass, and Isotopes of Elements • Chemical Bonding and Molecules • Chemical Reactions • Properties of Water • pH and Acid/Base Balance Chemical Reactions Essential Chemistry for Biology CHAPTER 2 • Elements and the Periodic Table • Atoms and Subatomic Particles • Atomic Number, Mass, and Isotopes of Elements • Chemical Bonding and Molecules • Chemical Reactions • Properties of Water • pH and Acid/Base Balance Water’s Life-Supporting Properties • Cohesion • Adhesion • Capillary action • Surface tension • Moderates temperature change • Less dense as a solid • Universal solvent QuickTime™ and a decompressor are needed to see this picture. Water Can Dissolve A Large Number Of Substances Table salt (NaCl) in water • Water surrounds ions and polar molecules, pulling them apart from each other and dissolving them. Substances that dissolve in water are called hydrophilic. • Water cannot dissolve non-polar (hydrophobic) molecules like oil, fat, and grease. • Since the majority of substances on earth are hydrophilic, water is called the universal solvent. Figure 2.16 Essential Chemistry for Biology CHAPTER 2 • Elements and the Periodic Table • Atoms and Subatomic Particles • Atomic Number, Mass, and Isotopes of Elements • Chemical Bonding and Molecules • Chemical Reactions • Properties of Water • pH and Acid/Base Balance Water Breaks Can Break Apart to Form Ions H2O water H+ hydrogen ion + OHhydroxide ion • When liquid water is pure, it has an equal number of hydrogen and hydroxide ions (H+ = OH-). Pure water is neutral. • Some substances, when added to water give off H+ and create acidic conditions (H+ > OH-). These substances are acids. • Other substances when added to water give off OHand create alkaline or basic conditions (H+ < OH-). These substances are called bases or alkali. • Organisms cannot survive in acidic or basic conditions because their chemicals are broken apart The pH Scale Is Used to Measure Acidity or Basicity Buffers • Substances called buffers can inhibit pH change when part of a water solution • Buffers soak up added H+ or OH- like chemical sponges • Humans have bicarbonate buffer in their blood to prevent pH change when eating and drinking. Essential Chemistry for Biology CHAPTER 2 • Elements and the Periodic Table • Atoms and Subatomic Particles • Atomic Number, Mass, and Isotopes of Elements • Chemical Bonding and Molecules • Chemical Reactions • Properties of Water • pH and Acid/Base Balance