Chapter 8 Reaction Rates and Equilibrium Spencer L. Seager Michael R. Slabaugh
advertisement

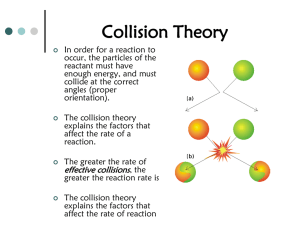
Spencer L. Seager Michael R. Slabaugh www.cengage.com/chemistry/seager Chapter 8 Reaction Rates and Equilibrium Jennifer P. Harris LEARNING OBJECTIVES/ASSESSMENT When you have completed your study of this chapter, you should be able to: 1. Explain ENTROPY and how this works with the classical energy changes of exothermic/endothermic to allow spontaneous reactions. 2. Explain reaction rates in general (non-quantitative) terms. 3. Use the concept of molecular collisions to explain reaction characteristics. (Section 8.3; Exercise 8.20) 4. Represent and interpret the energy relationships for reactions by using energy diagrams. (Section 8.4; Exercise 8.26) 5. Explain how factors such as reactant concentrations, temperature, and catalysts influence reaction rates. (Section 8.5; Exercise 8.30) 6. Relate experimental observations to the establishment of equilibrium. (Section 8.6; Exercise 8.38) 7. Write equilibrium expressions based on reaction equations, and use equilibrium constants in a qualitative way to understand equilibria. (Section 8.7) 8. Use Le Châtelier’s principle to predict the influence of changes in concentration and reaction temperature on the position of equilibrium for a reaction. (Section 8.8; Exercise 8.52) ENTROPY • Entropy is a measurement or indication of the disorder (or randomness) of a system. The more disorderly or mixed up a system is, the higher its entropy and the more favorable to develop. You can always replace the word “randomness” for entropy to make a statement easier to understand. • By contrast heat energy is referred to as enthalpy. • If enthalpy increases, heat is released (exothermic) which also is favorable to a process occurring. Examples of changes in Entropy Predict which of the following has an increase in entropy and where the change in entropy contributes to favorability. • The simple diffusion of a gas in air or a soluble substance in solution. • H2O(s) H2O(l) • H2O(l) H2O(g) • C6H12O6 (s) + 6 O2(g) 6 CO2(g) + 6 H2O(g) • NaCl(aq) + AgNO3(aq) AgCl(s) + NaNO3(aq) FACTORS THAT INFLUENCE FAVORABILITY • Overall favorability are dependent on the sum of both entropy and enthalpy. In practical terms, this means exothermic and becoming more random. • If both are favorable, then the overall process is favorable. • If both are unfavorable, then the overall process is unfavorable. • Where entropy and enthalpy are competing in favorability, enthalpy generally wins out since enthalpy values are usually larger than enthalpy values. ENERGY CONSIDERATIONS • Although Entropy considerations are important, we will for the most part limit our overall energy considerations to heat energy (enthalpy). • Exothermic reactions tend to be favorable and are written as Reactants = Products + heat • Endothermic reactions tend to be unfavorable and are written as Reactants + heat = Products • Exothermic reactions can be spontaneous (occur without any obvious input of energy or stimulus) but usually require a kick start (energy of activation). E.g. H2 and O2 don’t react without a spark. • Even though endothermic reactions are unfavorable, they can still occur but energy must be put into the system in order for this to happen. E.g., ice can melt. ACTIVATION ENERGY • In some reaction mixtures, the average total energy of the molecules is too low at the prevailing temperature for a reaction to take place at a detectable rate. The reaction mixture is said to be stable. • For many stable mixtures, the addition of a small amount of energy starts the reaction which then continues without the addition of any more stimulus or energy from an outside source. • The small amount of outside energy needed to start a spontaneous processes is called activation energy. ACTIVATION ENERGY EXAMPLE • An ordinary kitchen match provides a good example of these concepts. The reactants in the match head are stable until the match is rubbed on a rough surface. The energy of rubbing provides the necessary activation energy to cause the match head components to react and the match ignites. Once ignited, the match continues to burn spontaneously until all the fuel of the match has reacted with oxygen in the air. EXOTHERMIC & ENDOTHERMIC DIAGRAMS • The difference between endothermic and exothermic reactions is clearly indicated by the following energy diagrams. Note that in exothermic reactions, the energy is lost as the reaction occurs. Hence, the products have less energy than the reactants. The reverse is true for endothermic reactions which gain energy and cause the products to have more energy than reactants. DIFFERENCES IN ACTIVATION ENERGY • Activation energy differences become quite obvious in energy diagrams as shown by the following illustrations: REACTION RATES • A reaction rate is the speed of a reaction. • Reaction rates can be determined experimentally by measuring the change in concentration of a reactant or product and dividing the change by the time required for the change to occur, using the following equation: C C t C0 Rate t Δt • In this equation, ∆C is the change in concentration of a reactant or a product that occurs in a measured amount of time, ∆t. The value of ∆C is calculated by subtracting the initial concentration, C0, from the final concentration, Ct. HOW REACTIONS OCCUR Collision Theory • An explanation of how reactions occur is called a reaction mechanism. A reaction mechanism is often expressed as a number of processes that must take place for reactions to occur. The following assumptions make up the concept of “Collision Theory”: • Assumption 1: Reactant particles must collide with one another in order for a reaction to occur. • Assumption 2: Reactant particles must collide with at least a certain total energy if the collision is to result in a reaction. • Assumption 3: In some cases, colliding reactant particles must be oriented in a specific way if a reaction is to occur. HOW REACTIONS OCCUR (continued) • WHY COLLISIONS ARE NECESSARY • Reactant particles must collide if they are to react (assumption 1). With few exceptions, molecules cannot react with each other if they do not come in contact. During collisions, some bonds are broken, atoms are exchanged, and new bonds form. • WHY MINIMUM COLLISION ENERGY IS NECESSARY • The requirement that some bonds of reactant molecules must break if a reaction is to occur makes the requirement of minimum collision energies valid. This explains why hydrogen and oxygen don’t automatically explode without a spark. MOLECULAR ORIENTATION • Orientation effects are related to which side or end of a reacting particle actually contacts another particle during a collision. • The orientation of reacting particles is not important in some reactions such as those between reacting ions in a solution. For example, Ca2+ ions react with CO32- in solution to form solid CaCO3, which is insoluble and settles out of the solution. Both ions can be considered to be spherical charged particles, so their orientation toward each other when they collide does not influence the reaction rate. • Orientation is generally VERY important in organic and biological reactions where the molecule structures are very complex. FACTORS THAT INFLUENCE REACTION RATES • Reaction rates are influenced by a number of different factors, including: • the nature of the reactants (includes enthalpy, entropy, orientation, activation energy) • the concentration of the reactants, • the temperature of the reactants, • the presence of catalysts or inhibitors. THE NATURE OF THE REACTANTS • Reactions between oppositely-charged ions in solution occur almost instantaneously. This is because the ions are strongly attracted to each other because of their opposite electrical charges. THE NATURE OF THE REACTANTS (continued) • Reactions between covalently-bonded molecules in which covalent bonds have to be broken often take place slowly. This is partially because covalent bonds are relatively strong and must first be broken (activation energy is high) and partially because they generally require a specific orientation when colliding. • Other characteristics of reactants such as their physical state (gases, liquids or solids), their molecular sizes, and whether or not they are polar are also important influences of some reaction rates. THE CONCENTRATION OF THE REACTANTS • The requirement for a collision to occur between reactant molecules before a reaction can take place accounts for the reactant concentration influence on reaction rates. • If a reaction occurs between A and B molecules, and a reaction mixture contains mostly A molecules, most collisions participated in by A molecules will be with other A molecules and the reaction rate will be low. THE CONCENTRATION OF THE REACTANTS (continued) • The reaction between a solid piece of iron and oxygen gas takes place slowly in part because only iron atoms on the surface can collide with oxygen molecules. The effective concentration of iron is low. However, wire wool and finelydivided iron powder rust much more rapidly because the surface area and effective concentration is much greater. THE TEMPERATURE OF THE REACTANTS • The effect of temperature on reaction rates can also be explained using the concept of molecular collisions. • An increase in the temperature of the reactants corresponds to an increase in the velocity and the kinetic energy of the molecules. • An increase in velocity will increase the number of molecular collisions that take place in a fixed amount of time and will thus increase the reaction rate. • An increase in the kinetic energy of the colliding molecules will increase their velocity. Therefore there will be an increase the strength of collisions and the number of molecules with the required minimum activation energy. THE PRESENCE OF CATALYSTS • Catalysts are substances that speed up chemical reactions without being used up in the reaction. • One explanation for catalytic behavior is that catalysts provide an alternate reaction pathway that requires less activation energy than the normal pathway. We could say that when reactants are bound to the catalysts, that the catalyst weakens the bonds in the reactant making it easier to break the bonds. • Another explanation proposes that solid catalysts and enzymes provide a surface on which reactant molecules adsorb with favorable orientations to each other. Adsorbed molecules with favorable orientations are located close enough to each other to react rapidly. THE PRESENCE OF CATALYSTS (continued) THE PRESENCE OF INHIBITORS • An inhibitor is a substance that decreases reaction rates. • Inhibitors will be introduced in more detail when studying factors that affect enzyme activity (Chapter 20). • The characteristic function of many poisons and some medicines is to inhibit one or more enzymes and to decrease the rates of the reactions they catalyze. • Some substances normally found in cells inhibit specific enzyme-catalyzed reactions and thereby provide a means for the internal regulation of cellular metabolism. • Inhibitors are not necessarily bad things. They can help regulate reactions so they aren’t too fast. CHEMICAL EQUILIBRIUM • All chemical reactions can (in principle) go in both directions and products, located to the right of the arrow, can react to form reactants, located to the left of the arrow. This condition is indicated by the use of a double arrow pointing in both directions as shown below: H2(g) + I2(g) 2HI(g) • When the reaction rate toward the right is equal to the reaction rate toward the left, the reaction is said to be in a state of equilibrium. CHEMICAL EQUILIBRIUM (continued) • When a reaction is in a state of equilibrium, the concentrations of reactants and products remain constant as time passes. • The unchanging concentrations of reactants and products in a reaction at equilibrium are called equilibrium concentrations. THE POSITION OF EQUILIBRIUM • The position of equilibrium is an indication of the relative amounts of reactants and products present in a reaction mixture at equilibrium. • The position is said to be to the right when the amount of product is significantly more than the amount of reactant. • The position is to the left when more reactant is present than product. reactant left ⇌ product right An equilibrium we know about Alka seltzer and vinegar forms carbon dioxide and water NaHCO3 + HC2H3O2 H2O + CO2 + NaC2H3O2 HCO3- (aq) + H+ (aq) H2O(l) + CO2(g) Position is to the right. Or the reverse reaction (as in carbonated beverages) H2O(l) + CO2(g) HCO3- (aq) + H+ (aq) Position is to the left. MATHEMATICAL REPRESENTATION OF POSITION OF EQUILIBRIUM • The position of equilibrium can be represented mathematically by using the concepts of an equilibrium expression and an equilibrium constant. • Both concepts will be initially represented using the following hypothetical equilibrium: aA + bB ⇆ wW + xX • In this expression, the lower case letters represent the stoichiometric coefficients of the reaction and the upper case letters represent the formulas of the reacting substances. EQUILIBRIUM EXPRESSIONS • The equilibrium expression for the general equation on the previous slide is written as follows: W X K a b A B W X • In this equation, the brackets,[ ], stand for molar concentrations of the reactants, A and B, and the products, W and X. It is seen that each reactant concentration is raised to a power equal to the stoichiometric coefficient of that reactant in the equilibrium equation. EQUILIBRIUM CONSTANT • The K in an equilibrium expression is called the equilibrium constant. • As long as the temperature does not change, it has a constant value because none of the concentrations used to express it change with time once equilibrium is established. • A relatively large value for K indicates that the equilibrium position is toward the right or products side of the equilibrium. • A small K indicates an equilibrium position toward the left or reactant side of the equilibrium. • We will deal with the equilibrium constant in a general way as follows. products K reactants THE RANGE OF K VALUES • The values for K that have been found experimentally range between wide extremes. • Some, such as, K = 1.1 x 10-36, are so small that for all practical purposes an equilibrium mixture would contain only reactants and the equilibrium position is extremely far to the left. Reactants are favored when K << 1. • Others, such as K = 1.2 x1040, are so large that for all practical purposes an equilibrium mixture would contain only products and the equilibrium position is extremely far to the right. Products are favored when K >> 1. Equilibrium Constants for Acids • • • • Acetic Acid HC2H3O2 Sulfuric Acid H2SO4 Carbonic Acid H2CO3 Phosphoric Acid H3PO4 1.8 x 10-5 1.0 x 103 4.2 x 10-7 7.1 x 10-3 • Based on these numbers, where are products (or reactants) favored? • Rank these from strongest to weakest (most to least dissociated). • Does your answer sound correct in terms of how we defined Acetic Acid and Sulfuric Acid earlier in that acetic acid was a weak acid and sulfuric acid was a strong acid? FACTORS THAT INFLUENCE THE POSITION OF EQUILIBRIUM • According to Le Châtelier's principle, the position of equilibrium shifts in response to changes made in the equilibrium. • The factors that will be considered are: • concentrations of reactants and products • reaction temperature • catalysts • In general, Le Châtelier's principle predicts a shift away from the side to which something (including heat) is added and toward the side from which something is removed. An equilibrium we know about Alka seltzer and vinegar forms carbon dioxide and water NaHCO3 + HC2H3O2 H2O + CO2 + NaC2H3O2 HCO3- (aq) + H+ (aq) H2O(l) + CO2(g) What would happen to the equilibrium if we add more vinegar? H2O(l) + CO2(g) HCO3- (aq) + H+ (aq) What would happen to your cola if we allowed carbon dioxide to escape? FACTORS THAT INFLUENCE THE POSITION OF EQUILIBRIUM (continued) • When the temperature of a reaction is decreased, heat is removed. • If the reaction is endothermic, heat is a reactant and the position of equilibrium will shift toward the reactants. • If the reaction is exothermic, heat is a product and the position of equilibrium will shift toward the products. • Consider H2O(s) H2O(l) • Catalysts cannot change the position of equilibrium because they lower the energy barrier for both the forward and reverse reactions; therefore, catalysts speed up both forward and reverse reactions and cannot change the position of equilibrium. Le Châtelier's PRINCIPLE EXAMPLE • Consider the following endothermic reaction at equilibrium: heat + 4NO2(g) + 6H2O(g) 7O2 (g) + 4NH3(g) • How would the equilibrium shift under each of the following situations? • If an equilibrium mixture was heated • If some NO2 was added to an equilibrium mixture • If some NH3 was removed from an equilibrium mixture • If a catalyst were added Le Châtelier's PRINCIPLE EXAMPLE • Consider the following endothermic reaction at equilibrium: heat + 4NO2(g) + 6H2O(g) 7O2 (g) + 4NH3(g) • If an equilibrium mixture was heated, the equilibrium position would shift toward the right to try to use up the added heat. • If some NO2 was added to an equilibrium mixture, the equilibrium position would again shift toward the right to try to use up the added NO2. • If some NH3 was removed from an equilibrium mixture, the equilibrium position would again shift toward the right in an attempt to replace the NH3 that was removed. • If a catalyst were added, the equilibrium position would not change.