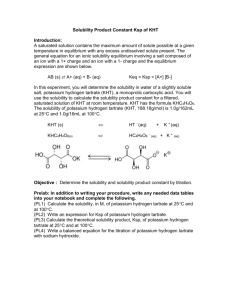
Solubility Product Constant of an Organic Salt Note : Objective :

Solubility Product Constant of an Organic Salt
Note :
The solubility of potassium hydrogen tartrate (KHT, 188.18g/mol) is
1.0g/162mL at 25C and 1g/16mL at 100C.
KHT (s)
K + (aq) + HT (aq)
Objective : Determine the solubility and solubility product constant by titration.
Prelab Questions :
(PL1) Calculate the solubility, in M, of potassium hydrogen tartrate at 25C and at
100C.
(PL2) Write an expression for Ksp of potassium hydrogen tartrate.
(PL3) Calculate the theoretical solubility product, Ksp, of potassium hydrogen tartrate at 25C and at 100C.
(PL4) Write a balanced equation showing the titration of potassium hydrogen tartrate solution with NaOH;
Procedure :
(1) Weigh out ~2.0g of finely powdered potassium hydrogen tartrate into 250.mL beaker.
(2) Add 150.mL of distilled water and stir well.
(3) Using a vacuum filtration, filter the solution into a clean, dry Erlenmeyer flask with a side arm.
(4) Record the temperature of the filtrate.
(5) Pipet out 25 mL of (4) into a 250.mL Erlenmeyer flask.
(6) Put ~3 drops of phenolphthalein indicator to (5)
(7) Prepare a buret with ~0.04M NaOH solution. (Make sure to write down the actual concentration.)
(8) Titrate (7) with NaOH until you get a permanent, faint pink solution.
(9) Repeat (5) to (8) for a second determination.
Calculations :
(C1) Calculate the amount of NaOH used in the titration.
(C2) Calculate your solubility of potassium hydrogen tartrate in M.
(C3) Calculate your solubility product, Ksp, of potassium hydrogen tartrate.
Post lab Questions :
(Q1) What are you assuming the filtrate is, unsaturated, saturated, oversaturated, or supersaturated?
(Q2) What would happen to the solubility and Ksp(that you calculated) if you started with 0.000001g of solid potassium hydrogen tartrate instead of 2.0g but you did n’t know it?
Summary :
Theoretical and your solubility of KHT
Theoretical and your Ksp of KHT