PART TWO Statistical Physics Chapter III:Statistic Distributions for ideal gases
advertisement

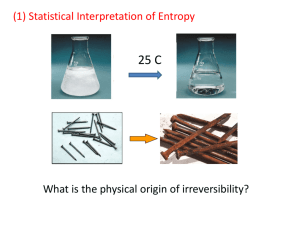
PART TWO Statistical Physics Chapter III:Statistic Distributions for ideal gases • 32 Statistics Regularities. Distributions, Most Probable Distributions (统计调节。分布,最大概率分布) • The main objective of statistical physics: • 1) to establish the behavior laws for macroscopic quantities of a substance. • 2) to offer a theoretical substantiation(证实) of thermodynamic laws on the basis of atomic and molecular ideas. Basic Methods • Condition : a system consisting of a large number N of molecules(由微观极大数目的粒子构 成). • Classical: using Newton’s classical mechanics(经典力学) to describe the state of the system; • Quantum: using quantum-mechanical description, the ideal of wave mechanics. Newton’s classical mechanics (经典力学) • Ignoring: intramolecular(分子内) structure, to visualize a molecule as a point or particle. • The equation of Newton’s motion for each of the N particles. dvi mi Fih ih dt • Fih:i’th与h’th分子的作用力;vi: velocity. • 求和存在的问题:1)要知道作用力或空间相关的作用势; 2)要知道6N个初始条件:每个分子的三维坐标与动量。 3)假设上述条件已知,求和计算分子的路径。 困难与解决方法 • 数学计算上的求和的难度,使其几乎不可能。 因为系统的粒子数达到1025m-3。 • 即使知道了粒子的路径和运动方程,也未必能 提供以系统作为一个整体有用的信息。 • In a system consisting of a great number of particles new purely statistical or probability laws take effect that are foreign to (不适合于) a system containing a small number of particles. Statistical Method • Assumption: It is possible to measure rapidly the energy of each molecule of a gas. The results of such measurement are present graphically in the Figure. • The axis of the abscissa(横坐标轴) is subdivided into equal sections each 0 long, and 0 is sufficiently small enough. All energies in l0~(l+1)0 is assumed to be equal to l0. Energy Distribution Function and “ Boxes” • The relative number of molecules in the range l0~(l+1)0 is denoted by n(l): n(l ) N (l , l 1) N • N is the total number. n(l) is “energy distribution function for molecules” or “energy distribution” • To divide the x-axis into longer unequal segments “Boxes” • The number of molecules with lower or higher energies is very small.(能量差别不大的分子属一个盒) Cell • A box is a larger unit which contains several cells: molecules have the fully same energy. • A box which energy l0~(l+m)0, • m0 is the length of a box, which varies little. • Actually, all the histograms(矩形高度) will be close to some averaged histogram and large deviations from it will be rare. Microstate 微观状态 • To describe the state of a gas at some moment of time: Microstate • Classical mechanics: “coordinates(坐标) and velocity” • 设粒子的坐标与势能相关,而动能与速度相关: mi (v xi2 v yi2 v zi2 ) 2 U ( xi yi zi ) •经典力学用坐标和动量描述粒子的“微观运动状态”。某一 时刻,整个系统的N个粒子构成了一个“微观状态”,如前 述的能量-粒子柱形图为能量分布图,或“状态分布”图。下 一个时刻,N个粒子的能量(坐标及动量)发生了变化,微观状 态也发生了变化。 • 一个气体分子平移运动的平均动量为 mv 2 3 kT 2 2 • 整个系统气体分子的平均能量为 l N (l , l 1) l n(l ) l l N • It is possible to find the mean values of any energy function if the “momentum distribution” are know. • 一个宏观的热力学系统用P、V、T、S描述。在一个 状态下(平衡或不平衡),均可用统计的微观状态描 述。即一个不变的热力学状态对应着许多的微观状 态,其数目被称之为“微观状态数”。 Basic physical postulate of statistical physics • “the greatest number of microstates of the most probable distribution and is equivalent to the equilibrium state of thermodynamics”.------两者相同点 • Thermodynamics assumes that a system remains in a state of equilibrium indefinitely long, but statistical physics predicts there existence of fluctuations(涨落) spontaneous(自发) and rare deviations from the equilibrium state. ------两者不同点 统计物理学关心的问题 • The problem of finding the most probable distribution for ensembles of non-interacting particles or for ideal gases. 33. -Space. Boxes and Cells • 借助于相-Space(六个坐标的空间)的概念导出统计分布。 • -Space : x,y,z, (ksai),(eta),(zita). System has N points. • This six-dimensional surface is specified by the equation: 2 2 2 2m U ( x, y , z ) •The concept of the phase volume(相体积) in the -Space is introduced by the expression: d dxdydzddd •Subdivided into(细分) the volume in the configurational space and in the momentum space. (构型空间和动量空间) dV dxdydz dV p ddd • It might be convenient to select the spherical layers(球壳层): dV=4rdr2, dVp=4pdp2. • 若简化为一维的运动: 2 2m mgx •相空间用代表点dxd 表示. 一个抛物线上的代表点能量相同。两个分子碰撞, 改变了各自的抛物线轨迹,但总能量不变。 Boxes in the -Space • The qi and pi are applied to represent coordinate and momentum. It is not homogeneous (均匀的) in all the space. • In phase volume d, the number of representative points is dN. The density is (qi,pi) = dN/d. • A postulate is introduced: the distribution function for the -Space, (qi,pi), depends only on the particle energy and not on qi and pi individually. • The -Space is subdivided into “boxes” by carefully drawing the hypersurfaces of “constant energy”. This energy layer is sufficiently thin that the representative points代表点confined in the layer have the same energy . 统计物理解决问题举例 • 一个三能级系统,0, 20, 30中,每个能级cells (原胞)有6个空位,共有6个完全相同的粒子,总 能量为120,每个空位只能放一个,粒子如何分布? • 粒子可以采取的分布方式为: 上图为粒子微观状态的表现,下图为分布函数,四种 微观状态出现的数目分别是1,6×15×6, 153, 202。 34 Bose-Einstein and Fermi-Dirac Distributions • Subdivision non-equidimensional energy boxes and equidimensional cells. • The ith energy box: having an energy i ,gi cells, Ni representative points. • How do these representative points distribute among the cells.? • Principle: any arrangement of representative points in the cells to be equiprobable. 等概率的 • The distribution is realized by the most probable distribution,--- the equilibrium state. Two Hypotheses 两个假设 • 1. All particles of one kind are absolutely identical to one another (所有粒子为全同). • 2. These particles differ slightly just as producting-line(生产线) identical parts produced in a factory differ from one another. • Both of above: “ particles of one kind are identical ” 微观状态的经典和量子描述 • N个全同粒子构成的体系,任意交换两个粒子的坐标和 动量时,经典力学认为其微观状态不同。因为经典力 学认为其运动轨迹是可以被跟踪的、每个粒子原则上 是可以被识别的。 • 量子力学认为,任意交换两个粒子的,其微观状态相 同。因为量子力学不可跟踪粒子的运动轨迹,运用的 是测不准原理和几率分布。 • 一个柱形图在量子力学条件下为一个微观状态,---此 为量子力学的“全同性原理”,全同粒子不可分辨; 而在经典条件下为多个微观状态,粒子可以分辨。 粒子的量子性 • 自然界中有两类量子粒子:fermion and boson • Fermions follow an important law: the Pauli exclusion principle • in a system of N identical fermions one cell in the -space can contain no more than one representative point. • in a system of N identical bosons one cell in the -space can contain any number representative points from zero to N. Statistical properties of the different particles • To illustrate the difference in the statistical properties of the different particles by a simple example: “Arrange two particles on three cells 1, 2, 3” For the classic particles, they are distinguishable 经典 玻色子 费米子 • Classical : 9 arrangements; • Bosons: 6 arrangements;Fermion: 3 arrangements. • How about Ni particles in gi cells? For the Boson: How to express? The ith box : Analyses Calculate Wi ( N i g i 1)! Wi N i !( g i 1)! • Wi: denoted as the number of different ways of arranging Ni particles in gi cells. • Two classes of objects: Particles & partitions • 粒子: Ni 和隔离物:gi-1 • 不同的排列方式可以分为两种交换: • (1)粒子和隔离物;(2)粒子和粒子。 • 因此,粒子和隔离物排列在一条线上,总的排列方式: • (Ni +gi-1)! :包含了全同粒子交换Ni ! 和隔离物交换(gi-1)! Boson系统的物理量: N Ni i ( N i g i 1)! W Wi N i !( g i 1)! • 要确定系统的最大几率分布,即确定W的最大值。 从数学上看,确定lnW较为方便。定义 = lnW ln W [ln( N i g i 1)! ln N i ! ln( g i 1)!] i [( N i g i ) ln( N i g i ) N i ln N i g i ln( g i )] i 利用了近似等式: ln Ni ! Ni (ln Ni 1) 求 的极大值 • 两个必要条件是:the total number of gas particles and the total energy of the gas are fixed. • 气体的分子总数一定;气体的总能量一定。用公式表示为 Ni N , i N i i U i 使用拉格朗日多项式变分的原理,使函数 = +N - U 的变分为零。得到 ln( N i g i ) ln N i i 0 N i The most probable number of particles in a cell is: Ni gi e i 1 (34.6) Fermi-Dirac distribution, Fermion (费米子) • Bose-Einstein distribution are specified by gi g i i N, U i e i e 1 1 •Fermions are considered. For the ith energy box with a number of cells gi, and a number of particles Ni (Ni < gi), the different ways of distribution differ from each other only in that some cells are occupied by one particle and some cells are empty---permutations(置换) of empty cells i i • 图中,一种分布为一个微观状态,此分布的微观状态数 目为空胞的置换次数。空胞数为(gi-Ni), 总置换数gi!, 总的粒子数置换数Ni!,总空胞置换数(gi-Ni)!微观状态为 Wi gi! N i !( g i N i )! N个粒子的排列方式是: W i gi! N i !( g i N i )! 微观状态数W的极大等价于的极大 [ gi ln( gi ) Ni ln Ni ( gi Ni ) ln( gi Ni )] i 求的极大得到 • 同理利用Lagrangian方法,使函数的微分为零: ln N i ln( g i N i ) i 0 N i 可以得到在Box上的粒子数: Ni gi e 1 l 并由此可以得到费米总粒子数的分布和能量公式: i i gi e l 1 g i i e l 1 N, U 35. The Boltzmann Principle • Two statistical distributions are known, but the meaning to be imparted(赋予) . • The important is to know the meaning of two parameters and . Make physical postulate: •1) W=W1·W2······Wn • =1+2+······+n • is a extensive quantity • 2) In an isolated system, and in “平衡态” • Thermodynamics: • Statistical Physics: •极大时的熵S对应着热力学系统的平衡态。 • 极为自然的问题: 和S 之间是否存在联系? •These arguments make it reasonable to postulate that with a degree of accuracy up to a constant multiplier thermodynamically defined entropy coincides(一致) with the quantity . 意义 Boltzmann consider this situation and thought that there must be some internal connection between them. He applied a multiple constant to establish an equation: S k ln W , 23 k 1.38 10 J / deg ------The Boltzmann Principle • Boltzmann endue(赋予) the entropy an statistical meaning . 宏观的熵其微观含义如何? • It is convenient to use another definition form. • 在统计物理中,常常使用更为方便的表达方式: S ln W (35.1) •Here , the entropy is assumed to be a dimensionless quantity. Since the product TdS must have the dimension of energy, then the temperature must be in the energy units. •即:熵S变成无维度的,温度变成能量单位的。 •The entropy of a system in a state of equilibrium is S max ln Wmax 熵的物理意义 • Boltzmann: the increase in entropy in an equalization process is the result of the system passing from a less probable states to the most probable state. 同一个物理系统,当它从完全混乱状态有序状态时,例如 无序排列的液体的水在温度不变的条件下分子结晶成冰, 熵如何变化? 例:一个红色墨滴滴入白色清水中,红色分子逐渐 扩散至均匀状态,其过程为熵增加。可以认为分子熵减小。 从有序变为无序。熵增加,反之,则为 因此,熵的统计意义就是分子的“混乱程度”. 等温分子的扩散,熵增加,有“虚的”吸热。(对应S1) About an irreversible process: • What is the fundamental difference between the statistical interpretation and the thermodynamic interpretation? • From thermodynamics: a reverse process is impossible by definition. • From statistical physics: a transition from the most probable distribution to the less probable. • (除非由于涨落的影响,且涨落会很大。) The discuss of distribution • The Boltzmann principle can be used to find the meaning of two parameters. By the formulae: i gi e l 1 N, i g i i e l 1 U The upper sign pertains to the Bose distribution, and the lower to the Fermi distribution. The entropy S is related with both N and U. •同一个BOX内的所有CELLS都是等价的,每个粒子占 据不同cell的概率完全相同。Cells 越多,粒子占据该box 的概率越大。为了表示粒子占据Cells的概率,定义了: Occupation Number “占有数”函数 • 粒子在一个cell上占据 的概率可以表示为: ni 1 e 1 i •由于该函数对费米分布来说是一个分布函数,但对玻色 分布来说有可能是大于1 的粒子数的函数:ni = Ni / g i , 故将其称之为粒子的“占有数”函数。作用? •首先,利用占有数函数可以对熵函数进行化简。在玻色 系统中,熵的表达式是: S [( N i g i ) ln( N i g i ) N i ln N i g i ln( g i )] i g i [( ni 1) ln( ni 1) ni ln ni ], (35.5) i her , N i g i ni 熵的化简形式(玻色分布) • 将玻色分布的ni代入得到: i S g i ln( 1 e i 1 e i •其中: ni 1 e , ni 1 i i e 1 e 将两个参量看作常数,得到: i i S N U g i ln( 1 e i i ) 1 1 1 e i ) (35.7) 熵的化简形式(费米分布) S [ g i ln( g i ) N i ln N i ( g i N i ) ln( g i N i )] i g i [ni ln( ni ) (1 ni ) ln( 1 ni )] (35.8) i • 将费米色分布的ni代入得到 i S g i ln( 1 e i 1 e i i ) 得到 i S N U g i ln( 1 e i ) (35.10) 熵的统一表达式 i S N U g i ln( 1 e ) (35.11) i • Here, minus bosons; positive fermions. • Important emphasize: • 1) 式(35.5)和式(35.8) 适用于平衡与非平衡态。 • 2)式(35.7) 和式(35.10)仅适用于平衡态,因为 在公式中包含了仅适用于平衡态时的极大熵 和极大占有数n1,n2,…,ni…以及确定平衡时熵 的两个参量与------热平衡的基本值。 The meaning of the parameters and • Compare Eq(35.11) with the expression dS in thermodynamics. • 分析方法:定gi, i为常数。 • 1) 不变,S对求导; • 2) 不变,S对 求导。 • gi和I将与气体的容器尺寸相关,即与V相关。 • 假设无任何外场的作用, 即式(35.11)不再包含 其他项,求导设定V不变,则 and 的偏导数 i S N U g i ln( 1 e ) (35.11) i S N U g i i U ( ) ,V ,V ,V i 1 e i S N ,V U ,V ,V (35.12) gi S N U N ( ) i e 1 ,V ,V ,V i N U ,V ,V (35.13) and 的值 S S dS ( ) ,V d ( ) ,V d • 将式(35.12)和式(35.13)代入得到: N U N U dS ( ) ,V d ( ) ,V d ( ) ,V d ( ) ,V d dSV dN dU 已知: dU TdS PdV dN 1 P 1 dSV dU dV dN dU dN T T T T T 1 1 ( ), ( ) T kT T kT • 关于单位:玻耳兹曼常数k是微观量,粒子数N是 宏观量,可以将此微观量转化为宏观量: • Nak = 6.023×1023×1.38×10 –23 = 8.31 (J K-1mol-1) • 在附录中R = 8.314 (J K-1mol-1); k = R/NA. • 理想气体的物态方程:PV = nRT = NkT. • 其中,N表示系统中的“粒子数”;n表示“摩尔 数”。 • Accordingly, in all following sections the chemical potential does not refer to one mole of substance, as in thermodynamics, but to one particle, so that therm = NA stat is true. ( s ,T)代替(,)在公式中: 统计物理学所表示的公式 • From the Eq.(35.11), the entropy is i S N U g i ln( 1 e ) (35.11) i TS N U T g i ln( 1 e ( i ) / T ) i 此时的i为一个粒子的能量,表示某个粒子所处的能 级。而熵是对所有这种能级求和。虽然我们已知了 是化学势,也知道在热力学中它是相变的推动力,然 而,在统计物理学中是否还有新的含义?长远的应用? •G = N;(为一个分子的化学势)。 •F = U – TS = G – PV,F – G=U – TS – G= U -TS- N • 得到公式: PV T g ln( 1 e ( ) / T ) i i i •最终,统计公式可以写为: Ni gi ( l ) / T gi e ( i ) / T N, 1 g i i ( l ) / T (35.20) U (35.21) i e e 1 1 参量和一旦确定,上式的物理意义会发生变化: i 仅仅是温度的函数, 已经确定;上左式中,化学势 是粒子数N和温度T的函数,而上右式中,内能是化 学势和温度的函数。可以推断:内能是粒子数N和温 度T的函数。 ------理想气体的焦耳定律。 • 进一步分析,每个粒子的内能U/N将仅仅是温 度的函数,从而成为强度量。V/N也将如此。 • 如果外加其他场强的话,系统如何变化? i is the function of intensive parameters, i.e. field intensities. In Conclusion • The B.E. distribution and F.D. distribution are derived by the box-cell method presupposing (预 先假定) that thermodynamic equilibrium state sets in. • The initial non-equilibrium particle distribution equilibrium distribution particles change their boxes to a equilibrium state. • Reason: N=cons. and particles interact with surrounding walls (thermostat). • Indeviation: both N and U are fixed, only ~T. 36. The Maxwell-Boltzmann Distribution • Question: How does classical particles distribute? • Let Box1 for N1, Box2 for N2, … • Box n for Nn, … . • • • • 任意两个粒子两两交换:N! 次 不考虑N1个粒子在BOX1中的交换; 不考虑N2个粒子在BOX2中的交换; 因此,将N个粒子填充到BOX 中的数目是: N! W ' Ni! 微观状态数 • 在一个BOX内,有gi个cells,Ni个粒子。由于 每个粒子均是可以被跟踪的,即可以被识别的。 因此,任意交换两个粒子为不同的微观状态。 Ni g • 在一个BOX内总的交换次数是多少? i 由此可以算出总的微观状态数目: Ni gi N! Ni W N ! gi i Ni! Ni! i i 运用Stirling公式,得到: N ln N [ Ni ln gi Ni ln Ni ] i • 使用拉格朗日变分公式: ( 1) N U 对Ni求导得到最后结果: N i g i e i i gie N i i g i i e U i 而量子的分布是: ni 1 e i 1 • If for any i the condition exp(i -)/kT >>1 is satisfied, the unity in the denominator can be ignored and we obtain the Maxwell-Boltzmann distribution: Ni gie ( i ) / kT ni N i / g i e ( i ) / kT •对于理想气体近似为实际气体的条件,就是: ni N i / g i e ( i ) / kT 1 In this rarefied gas, the average interparticle distances are large, so they cannot be confused---distinguishable. 在稀薄气体条件下,粒子之间的距离较远,不可被分辨的 “量子”与可以被分辨的经典粒子是完全一致的。 结论:稀薄浓度的量子粒子可 以近似为经典粒子处理 • The Boso-Einstein and the Fermi-Dirac distributions are valid for all particles, thile the Maxwell-Boltzmann distribution is approximately true in the limiting case of small occupation number. • The entropy of a gas in an arbitrary equilibrium or non-equilibrium state can be obtained in two ways: 熵的计算 S gi ni ln ni N i • If the Boltzmann formula S=lnW is used, the classical gas (36.2) would follow: S g i ni ln ni N ln N * i It is not true, otherwise, S* will be not an extensive quantity.------ we return to the Gibbs paradox. Gibbs 的预言 • Gibbs’ foresight is worthy of admiration, for as far back as the end of the nineteenth century he anticipated the present-day concept of the indistinguishability of particles. • 值得注意的是,玻耳兹曼分布应用的范围是有效的, 其不等式是成立的。即便当gi很大时,Ni也会很小or close to unity. • In these conditions, the Stirling’s formula becomes incorrect for Ni and gi. • An general Gibbs’ method can be applicable to ideal gses but also the systems of interacting particles. • Problem: Page 190 • 中文教材内容补充: • 在一个长为L,粒子数为N的容器内,粒子以波 动的形式运动。运动方式为: • 其运动方式为驻波。 驻波的波长为 = L/n. n为正整 数。定义波矢为k=2/ . 波矢具有两个传播方向,定义 2 kx nx , L nx 0,1,2,... 动量与能量 2 p k nx L 2 px 2 2 2 2 nx nx 2 2m mL 1 2 nx n y nz 2 2 2 ( px p y pz ) 2m m L2 L dnx dp x , ...... 2 L 3 V dnx dn y dnz ( ) dp x dp y dp z 3 dp x dp y dp z 2 h 2 2 2 2 2 2 作业:P228: 6.2, 6.4, 6.5 • 上式表示在一定动量空间内代表点(量子态)的 数量。在一维空间,一个代表点的体积(Ldp)是 h。三维空间一个代表点的体积是h3。量子态 与动量之间有直接的对应关系。 • 利用能量-动量关系 =p2/2m. • 动量有正负,能量是简并的。 • 相同能量而动量不同,为同一状 态。(能量---BOX,动量---Cell) 能量或状态是分立的,即在单位能量长度内其状 态数目用D(E)表示。故 D ( ) d V3 4p 2 dP h D ( ) 2V h3 ( 2m) 3 / 2 1 / 2 什么是波矢 k ? • 从量子的角度看,波矢对应着速度: • p = hk = mv • 电子从低能级跳到高能级 能量和动量均发生了变化。 横坐标表示动量的变化, 纵坐标表示能量的变化。 当一个粒子(电子)碰到一个高速振动的粒子 (离子)时,会获取能量和动量。 What is the concept of “Boxes” D ( ) d V h3 4p 2 dP D ( ) 23V ( 2m) 3 / 2 1 / 2 h • Here, D() is defined as the density of state. dN D( ) d dN is the number of energy in the range of ~ + d. How about D() ~ V, ~m, ~ ? What is the “box”? One box is one state(能量状态,不是 量子态), or one line in the figure, about one value of . • • • • 37. Transition to continuously Varying Energy. Degeneracy Conditions for Ideal Gases Three Statistical Distributions 1) the Bose-Einstein distribution 2) the Fermi-Dirac distribution 3) the Maxwell-Boltzmann distribution 1) N N i gi , 1 U e ( i ) / kT 1 gi i gi 2) N N i ( ) / kT , U ( ) / kT i i i e i e i 1 1 3) N N i g i e ( i ) / kT , U i g i e ( i ) / kT i i i i e ( i ) / kT i gi i i Discussion • In deriving the statistical distributions, the energy was a “discretely varying quantity” ------“Box”. • If it is suitable? In what degree? Size of the cell? • If the energy layers(boxes) are sufficiently thin, we can even replace above summation by integration. • How do we integrate? • By a new concept “phase volume” ----d=dqidpi • In this volume the particle number is dN. • If the volume of one cell is “a”, “g” weight factor dqi dpi d gi g g a a The meaning of g • For instance, the spin of a particle is s, the projection of the spin in any direction have 2s+1 different values(-s, -s+1, … s-1,s). In this case g = 2s+1. • The light quantum, photon, has not spin, but has two vibrational directions, g = 2. • The photon is Boson. • The electron, the Fermion, g = 2s+1 = 2. Three distributions g d g d 1) N ( ) / kT , U ( ) / kT a e a e 1 1 g d g d 2) N ( ) / kT , U ( ) / kT a e a e 1 1 g ( ) / kT g 3) N e d, U e ( ) / kT d a a • The important distinction(差异)。 • For the boson and fermion, a can be solved by the comparison with the experiment results of Cv. U Cv ( )V T The discussion of MaxwellBoltzmann distribution • The situation is quite different for the Maxwell-Boltzmann distribution. The chemical potential and the cell volume are presented in the same form: exp(/T)/a. U N But two others U N / T e d / T e d ( ) / T 1 ( e 1 ) d ( ) / T 1 ( e 1 ) d Distinction • The energy depends on and, consequently, (从而) on a。For the exact statistical Fermi-Dirac and Bose-Einstein distribution, the volume of a cell is not arbitrary: exactly by laws of nature and by experiment. • Impossible in the case of small occupation numbers, because of the phase volume of a cell acquires arbitrary value. 在此情况下,可以考虑用经典分布替代量子分布。 在什么情况下可以替代,标准如何? The criterion of validity of the Maxwell-Boltzmann distribution • In the case of mono-atomic ideal gas g d N ( ) / kT a e 1 p2 2 2 2 , 2m 2m 能量依赖于动量,细分球形动量空间得到: d V 4p dp V 2 (2m) d 2 3/ 2 1/ 2 已知Maxwell-Boltzmann 分布的有效性为: ( ) / T /T e 1, i.e. e 1 (including 0) 1 Na / T Na /T e e d g gV (2mT )3 / 2 1 Na / T Na e e d 3/ 2 g gV (2mT ) • 对于一个单原子气体,一个元胞的体积为 a = h3,(Planck’s constant), and g = 1 • The criterion of validity of the MaxwellBoltzmann distribution is /T N V h 8mT 2 3/ 2 h 1, i.e. n 8mT 2 3/ 2 1 The criterion are low density, high temperature, and large molecular masses m. 简并条件 • The reverse criterion 3/ 2 2 N h 1 i.e. V 8mT 3/ 2 h 3/ 2 n T (37.9) 8m • The MB distribution is inapplicable; • The gas obeys the BE or FD distribution. • The gas is then said to be “degenerate”(简并) 2 • Eq.(37.9) is known as the “degeneracy criterion” • For a gas: at low temperatures shows “quantum” and at high temperatures shows “classical”. Examples • For the ordinary atoms, N/V~1019cm-3, m ~ ( 10-23 to 10-24 )g, T<<10-1K as quantum. • For ordinary gases, the normal MB distribution is a good approximation down to rather low temperatures. • 随着低温技术的提高,最近几年统计物理学的主 要研究内容是极低温下气体所表现出的量子效应--“玻色凝聚”。 • 由于电子的质量仅为10-27g, 临界温度高达104K。