Jeremiah Jones FWLM HW4 Problem 1
advertisement

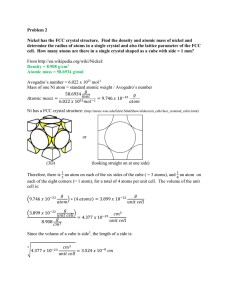
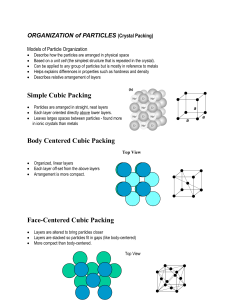
Jeremiah Jones FWLM HW4 Problem 1 When analyzing a material, there are many attributes which need to be considered. One very important characteristic of the material is the structure of the surface. The above picture shows two examples of a closed packed surface structure. One of the two examples is called an FCC structure, or face centered cubic structure, is an cubic atomic arrangement where the is an atom on each corner of the cube as well as an atom at the center of each of the cube faces/sides. This results in what is known as an “ABC” repeting pattern of atoms, as shown on the left side of the figure above. The other of the two examples is called an HCP structure, or hexagonal close packed structure. In this structure, instead of imagining a cube of atoms, imagine that each sequential row of closely packed atoms is shifted such that each atom of the shifted row fits into the empty space between atoms of the rows below and above. This results in a “AB” repetitive structure. Some examples of materials with FCC structure are Aluminum, Copper, Gold, and Nickel. Some examples of materials with HCP structure are Cadmium, Cobalt, and Zinc.