AWPPCE Bill Bardin Homework 9
advertisement

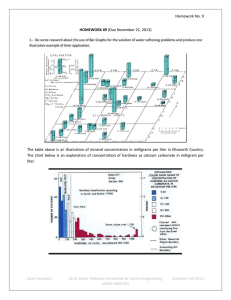
AWPPCE Bill Bardin Homework 9 9.1 USGS Chart showing the relative distribution of hardness from various test sites. http://water.usgs.gov/owq/chart.jpeg Graph showing mineral concentration in Ellsworth County Kansas. http://www.kgs.ku.edu/General/Geology/Ellsworth/05_gw.html 9.2 Cyanide, in its pure form, is extremely toxic to humans. There are many cyanide containing compounds that are in common use in industry today. Most urethanes, foams, soles, adhesives, coatings, etc., are produced using cyanide-containing compounds like isocyanates. Cyanide is a carbon atom triple-bonded to a nitrogen atom. Cyanide is found at low levels in nature. Chronic, long-term, inhalation exposure to cyanide primarily manifests itself in effects on the central nervous system. Cyanide is prevalent in gold production and, until relatively recently, was disposed of as a byproduct of the mining process. Gold processing facilities now process and recycle the waste streams from the mining process. Cyanide is reacted and converted to a non-toxic form achieving effective cyanide detoxification. 9.3 A reductive treatment for CR(VI) is called phytoremediation. Phytoremediation, “ is a multi-faceted approach towards Cr remediation. Plants contain the Cr by converting it to the less mobile Cr(III) (phytostabilization) and simultaneously reduce its toxicity. In addition, phytoremediation can be a removal technology, if Cr is sequestered in plant tissue and the plants are harvested. Another possible reductive treatment for Cr(VI) is microbial reduction. Microbial reduction, “microorganisms can catalyze redox reactions by a combination of several mechanisms, including enzymatic extra-cellular reduction, non-metabolic reduction by bacterial surfaces and intra-cellular reduction and precipitation.” A third reductive treatment for Cr(VI) is through a chemical reaction, this approach achieves both a reduction in Cr toxicity and removal of the Cr from aqueous solution. Chemical reduction includes naturally occurring reduction by soil oxides and natural organic material. Engineered chemical reduction technologies involve the addition or insitu injection of an electron donor such as hydrogen sulfide (H2S). http://www.engr.uconn.edu/~baholmen/docs/ENVE290W/National%20Chromium%20Files%20From%20Luke/Cr(VI)%20Handbook /L1608_C08.pdf 9.4 In recent years, rainwater harvesting has emerged as a water conservation tool and an alternative water supply source, especially in arid areas of the United States. Escalating costs of centralized water systems and well drilling, an increasing recognition of the beneficial qualities of rainwater and dwindling water supplies are contributing to this trend. Interest in rainwater harvesting has also been spurred by the prospect of rising municipal water demand resulting from the enormous population growth. Rainwater harvesting is nothing new. Humans have been harvesting rainwater since we first began to walk erect on this planet. Harvesting rainwater is only a small part of the entire water cycle, but it is of growing importance. It’s not just about harvesting the rainwater but efficient use of all our water resources. Many communities in the United States face serious threats to a safe, steady supply of water. These include a longstanding reliance on centralized water delivery systems that results in urban areas and agencies largely overlooking opportunities to integrate alternate local sources of water to meet their water supply needs. One of the biggest offenders is the unnecessary use of potable water for non-potable uses, such as irrigation and indoor toilet flushing. The continually increasing areas of impervious surfaces, parking lots driveways, sidewalks and buildings in our landscape result in storm water runoff carrying pollution to our rivers, lakes, and beaches. Although the problems of water supply and water pollution can be complex, practical solutions for both are available now, such as capturing and using rainwater from rooftops. I intend to look at this process as well as grey water recycling as viable alternatives to using treated water in applications that would be well served by non-potable water.