MEET THE PLATELET
K. Krishnan MD
Department of Internal
Medicine
Acknowledgements
My teachers at PGI,
Chandigarh,
Hammersmith Hospitals,
UK and U of Michigan,
Ann Arbor
American Society of
Hematology for images
LEARNING OBJECTIVES
Understand platelet development and function
Understand the classification of platelet
disorders
Understand the clinical manifestations of platelet
disorders
Understand the methods available to diagnose
platelet disorders
Understand the pharmacological agents used to
treat platelet disorders
PLATELET HEMATOLOGY
Platelet development and kinetics
Platelet tests
Clinical aspects of platelet disorders
Qualitative platelet disorders
Platelet function disorders
Congenital
Acquired
Quantitative platelet disorders
Thrombocytopenia
Thrombocytosis
Platelet therapeutics
PLATELET DEVELOPMENT
Small anucleate fragments formed from the megakaryocyte
cytoplasm
Characteristic discoid shape
Hematopoeitic stem cells are converted into MGKs by
exposure to the specific growth factor, thrombopoietin
Tpo initiates a maturation program
Amplifies the megakaryocyte DNA
Synthesis of platelet-specific proteins
Cytosketal elements, membrane systems and receptor
proteins are bulk produced
Platelet production begins when microtubules aggregate in the
cell cortex, elaborate pseudopodia
These pseudopodia develop into proplatelets
Platelets are assembled at the end of proplatelets
Microtubules deliver intracellular organelles into these
proplatelets
Platelets are released from the ends of proplatelets
PLATELET KINETICS
Platelets are produced in bone marrow by
megakaryocytes
MGKs produce platelets by cytoplasmic shedding
into bone marrow sinusoids
1000-5000 platelets per MGK
35k to 50k platelets per microl of whole blood per
day
Platelet life span 8-10 days
Removed from circulation by monocytemacrophage system
Determinants of megakaryocytopoiesis and thrombopoiesis.
Battinelli E et al. PNAS 2001;98:14458-14463
©2001 by National Academy of Sciences
EARLY MEGAKARYOCYTE
INTERMEDIATE STAGE MEGAKARYOCYTE
MATURE MEGAKARYOCYTE
PLATELET FUNCTIONS
Adhere to sites of vascular injury
Generate biological mediators
Secrete granule contents
Form multicellular aggregates
Serve as a nidus for plasma coagulation reactions
PLATELET FUNCTIONS
For these platelet functions,
Structural rearrangements
Utilize multiple membrane receptors
Bind small molecule mediators
Bind adhesive glycoproteins and constituents of vascular
endothelium
Activate a network of complex signaling pathways
HOW TO ASSESS PLATELETS
Automatic/Manual Platelet count
Peripheral smear
Bone marrow examination and specialised tests
Platelet function testing
PFA test/screening test
Specific tests using platelet aggregometry (many
methods/instruments)
Thrombin, Collagen, ADP, Arachidonic acid, Ristocetin
Antibody assays
CLINICAL FEATURES IN PLATELET
DISORDERS
Splenomegaly/Chronic liver disease
Petechiae or dry purpura
Begins in the dependent portions of the body due to venous
pressure-ankles and feet in an ambulatory patient
Occurs when platelet count decreases; not seen in disorders
of platelet function
Differentiate dry non-palpable purpura from palpable
purpura seen in vasculitis eg. Henoch-Schonlein purpura
Wet purpura- look in mouth, oral mucosa
Sign of severe thrombocytopenia
Denotes risk for significant hemorrhage
Excessive bruising
Seen in disorders of platelet function and number
CLINICAL FEATURES OF PLATELET
DISORDERS
HIGH PLATELET COUNT
Thrombocytosis
Symptoms due to high platelet count
Easy bruising
Bleeding due to platelet dysfunction
Thrombotic tendencies
TIAs
Erythromelalgia
Mild splenomegaly
BRUISING
PURPURA
PURPURA
PURPURA
Seen in dependent
areas of the body
Palpable purpura: Henoch-Schonlein Purpura
SCURVY
Arch Dermatol. 2010;146(8):938-938. doi:10.1001/archdermatol.2010.162
Date of download: 6/10/2012
Copyright © 2012 American Medical
Association. All rights reserved.
PLATELET FUNCTION DISORDERS
Defects of platelet-vessel wall interaction (disorders of
adhesion)
Defects in platelet- platelet interaction (disorders of
aggregation)
Defects in platelet- agonist interaction (TXA2, COX, Collagen, ADP)
Defects in cytoskeletal regulation
Storage pool deficiency
Quebec platelet disorders
Disorders of platelet secretion and signal transduction
Congenital afibrinogenemia
Glanzman’s thrombasthenia
Disorders of platelet secretion and abnormalities of granules
Von Willebrand disease
Bernard Soulier syndrome
Wiskott- Aldrich syndrome
Disorders of platelet coagulant-protein interaction
(membrane phospholipid defects)
Scott syndrome
INHERITED PLATELET DISORDERS
Rare, heterogenous group
Not often seen in clinical practice
Yet fascinating abnormalities that provide
insight into normal platelet biochemistry and
physiology
INHERITED PLATELET DISORDERS
Disorders of
Platelet membrane
Platelet granule packaging
Hereditary macrothrombocytopenias
Platelet signaling disorders
Platelet coagulant function disorders
PLATELET MEMBRANE DISORDERS
GLANZMAN’S THROMBASTHENIA
“Weak platelets”
Platelets carry out most of the functions
Platelet count is normal
Platelet morphology is normal
Platelets adhere normally to vascular
endothelium
Platelets secrete granules and perform normal
signalling functions
Platelets DO NOT AGGREGATE due to loss of
GpIIb/IIIa receptor
Normally this complex binds fibrinogen linked
into multicellular aggregates
PLATELET MEMBRANE DISORDERS
GLANZMAN’S THROMBASTHENIA
Inherited
Most are compound heterozygotes
Life long mucosal bleeding
Life long platelet transfusions
Recombinant Factor VII
Acquired
Rare, autoantibodies that bind to GpIIb/IIIa epitopes
Seen in ITP and in patients with normal counts
Steroids may not work
Immunotherapy/Rituxan may work
BERNARD SOULIER SYNDROME
Autosomal recessive
Gp1b deficiency or defect
Gp1b is the principal receptor for vWF
No functioning Vwf receptor
Platelets cannot adhere to vascular endothelium
Giant platelets and thrombocytopenia
Large size due to lack of interaction between actin binding
proteins in platelet cytoskeleton and cytoplasmic domain of
gp1b
Lack of gp1b bound sialic acid residues causes shortening
of platelet survival leading to thrombocytopenia
Platelet transfusions, DDAVP and fibrinolytic
inhibitors like EACA
WHAT IS THIS?
ACQUIRED QUALITATIVE PLATELET
DISORDERS
Drugs
Aspirin
Treat with platelet transfusions for severe bleeding
NSAIDs
Glycoprotein inhibitors like Abciximab
ADP receptor antagonists like Clopidrogel
Uremia
Toxic effects of uremia plasma, impaired plateletvessel wall adhesion and increased production of NO
Platelet transfusions ineffective
Treat with dialysis, DDAVP, conjugated estrogens
Myeloproliferative disorders
Myelodysplastic disorders
WHAT IS THIS?
How does it
happen?
PSEUDO-THROMBOCYTOPENIA
Pseudothrombocyt
openia secondary
to platelet
satellitism is
illustrated in this
image. Platelets
are shown to
adhere to the
cytoplasmic
membrane of two
of the PMNs
present on this
peripheral blood
smear. This
phenomenon is an
in vitro artifact
that occurs with
EDTA
anticoagulant.
Collection of the
blood specimen in
either sodium
citrate or heparin
corrects the
abnormality.
CLASSIFICATION OF THROMBOCYTOPENIA
Impaired or decreased production
Congenital
May –Hegglin anomaly
Bernard- Soulier syndrome
Wiskott- Aldrich syndrome
TAR
Congenital amegakaryocytic thrombocytopenia
Neonatal
Infective/viral
Drug induced
Acquired
Increased platelet destruction
Immune
ITP
Drug induced
HIT
Non-immune
Thrombocytopenia in pregnancy and pre-eclampsia
HIV
TTP
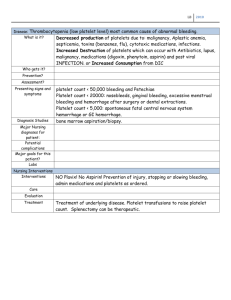
DIC
HUS
Drugs
Disorders related to distribution or dilution
Splenic sequestration
Kasabach-Merritt syndrome
Hypothermia
Loss of platelets- massive blood transfusion, extracorporeal circulation
THROMBOCYTOPENIA
Impaired or decreased platelet production
Megakaryocyte hypoplasia
Ineffective thrombopoeisis
Usually congenital and include
Fanconi anemia, thrombocytopenia with absent radii
(TAR syndrome), Wiskott- Aldrich syndrome, BernardSoulier syndrome, May Heglin anomaly, congenital
amegakaryocytic thromobocytopenia
Megaloblastic anemia
Miscellaneous
Viral
Marrow infiltration by malignancy, myelofibrosis
MAY-HEGGLIN ANOMALY
MAY-HEGGLIN ANOMALY
A
macrothrombocyte
is present in this
view. The PMNs
have blue
cytoplasmic
inclusions
bordering the cell
surface
membrane. These
inclusions contain
precipitated nonmuscle myosin
heavy chains
characteristic of
this group of
congenital
quantitative
platelet disorders.
Neutrophil
function in this
disorder is normal.
CONGENITAL AMEGAKARYOCYTIC
THROMBOCYTOPENIA
AR disorder causing bone marrow failure
Seen in infancy
Platelet count <20
Petechiae and physical anomalies
Develop aplastic anemia, MDS and leukemia
Stem cell transplantation is curative
Mutations in the c-mpl gene leading to loss of the
thrombopoietin receptor function
Loss of TPO receptor function causes reduction in
MGK progenitors and high TPO levels
ACQUIRED HYPOPLASIA
Drugs
Chemotherapy drugs
Zidovudine
Ethanol
Interferon therapy
Anticonvulsants
Antibacterial agents like chloramphenicol
INFECTION INDUCED THROMBOCYTOPENIA
Many viral and bacterial infections without DIC
Infections affect platelet survival and production;
immune mechanisms can also be at work
(Infectious mononucleosis, early HIV)
At times, bone marrow exam may be required for
occult infections
THROMBOCYTOPENIA
INCREASED PLATELET DESTRUCTION
Immune thrombocytopenic purpura
Acute
Disorder of children
Abrupt onset
Follows an infection usually nonspecific respiratory or GI
virus
Diagnosis is clinical
Most patients recover without treatment within 3 weeks
Severe cases can be treated with IVIG, platelet transfusions
and splenectomy
Occasionally seen in adults
THROMBOCYTOPENIA
INCREASED PLATELET DESTRUCTION
Chronic ITP
20-50 yrs of age
Females:males 2:1
Mucocutaneous bleeding, menorrhagia, recurrent
epistaxis or easy bruising
Immune mediated destruction of platelets
Autoantibodies against platelet glycoproteins
CLINICAL PICTURE OF ACUTE AND
CHRONIC ITP
Characteristics
Acute
Chronic
Age at onset
2-6 yrs
20-50 yrs
Sex predilection
None
Female over male 3:1
Prior infection
Common
Unusual
Onset of bleeding
Sudden
Gradual
Platelet count
<20
30-80
Duration
2-6 wk
Months to years
Spontaneous
remission
90%
Uncommon
Seasonal pattern
High in winter/spring None
Therapy
70% steroid
responsive
30% steroid
responsive
Splenectomy rare
Splenectomy
<45 yr 90% response
>45 yr 40% response
BONE MARROW IN ITP
MEGAKARYOCYTIC HYPERPLASIA
PhlWHWWegmasia Cerulea Dolens
Bawrham K, Shah T. N Engl J Med 2007;356:e3.WHA
HEPARIN-INDUCED THROMBOCYTOPENIA
(HIT)
Differs from other drug induced
thrombocytopenias
Thrombocytopenia never severe ie <20k
Not associated with bleeding but with thrombosis
Antibody to a complex of platelet specific PF4 and
heparin (anti-PF4/heparin)
Antibody activates platelets through the FcYR II
a receptor; also activates endothelial cells
Many patients exposed to heparin develop this
antibody though not all develop HIT and even
less develop HITT
HIT
Both standard heparin and LMWH can cause
HIT-former more common
Heparin exposure 5-10 days
Rarely HIT can develop several days after
heparin discontinued called delayed onset HIT
Diagnostic algorithm 4Ts
Thrombocytopenia
Timing of platelet drop
Thrombosis
oTher cause of thrombocytopenia not evident
CLINICAL TEACHING POINTS ABOUT HIT
Early recognition; HIT remains a clinical
diagnosis
Thrombosis can be arterial and/or venous
When HIT suspected, doppler legs
Anticoagulate when HIT suspected even in the
absence of thrombosis because of higher rate of
thrombosis (alternate AC followed by 3-6 months of
warfarin)
Risk of thrombosis persists for about 1 month after
diagnosis of HIT
Do not introduce warfarin alone in setting of HIT or
HITT as it may precipitate thrombosis especially
venous gangrene. Start after several days of alternate
anticoagulation
ALTERNATE ANTICOAGULANTS IN
HIT/HITT
Direct thrombin inhibitors
Argatroban
Lepirudin
Bivalirudin
Both approved in the US
Effective but not FDA approved
Antithrombin-binding polysaccharide
Fondaparinux
Effective but not FDA approved in the US
Anti-Xa
Danaproid
No longer available in the US
PREGNANCY AND THROMBOCYTOPENIA
You are asked to see a pregnant patient with
thrombocytopenia.
What is the differential diagnosis?
Differential diagnosis of thrombocytopenia in pregnancy
MAHA
Thrombocyto
penia
Coagulopath
y
HTN
Liver disease
Renal disease
CNS
Time of onset
ITP
------
Mild to severe
-------
--------
---------
---------
---------
Anytime
common in
first tri
Gestational
--------
Mild
-------
---------
---------
---------
---------
2nd-3rd tri
Preeclampsia
Mild
Mild to
moderate
Absent to mild
Mod- to severe
-------
Protein
Seizures
3rd trim
HELLP
Moderate to
severe
Mod to severe
Mild
Absent to
severe
Mod to severe
Absent to
moderate
Absent to
moderate
3rd trim
HUS
Mod to severe
Mod to severe
Absent
Absent to mild
Absent
Mod to severe
Absent to mild
Post partum
TTP
Mod to severe
Severe
Absent
Absent
Absent
Absent to
moderate
Absent to
severe
2nd- 3rd
trim
AFLP
Mild
Mild to mod
Severe
Absent to mild
Severe
Absent to mild
Absent to mild
3rd tri
NON-IMMUNE MECHANISMS OF PLATELET
DESTRUCTION
Thrombocytopenia in pregnancy and
preeclampsia
Gestational thrombocytopenia
Commonest cause
Usually mild
Healthy with no prior history of thrombocytopenia
Mechanism unknown
Return to normal a few weeks after delivery
NON IMMUNE CAUSES OF PLATELET
DESTRUCTION
Thrombocytopenia in preeclampsia and
hypertensive states in pregnancy
Thrombocytopenia occurs in about 15- 20% of
preeclampsia
Some have microangiopathic hemolysis, elevated
liver enzymes, and low platelet count-HELLP
syndrome
Thrombocytopenia is due to platelet destruction
Perhaps an underlying low grade DIC or ? Immune
process
Delivery is the treatment for this conditionthrombocytopenia will resolve in a few days post
delivery
MICROANGIOPATHIC HEMOLYTIC ANEMIA
(MAHA)
NON IMMUNE CAUSES OF PLATELET
DESTRUCTION
Thrombotic thrombocytopenic purpura
Triad of microangiopathic hemolytic anemia,
thrombocytopenia, neurological abnormalities
Sometimes the pentad- fever + renal dysfunction
Four types
Single acute episode
Recurrent episodes
Drug induced
Chronic relapsing-rare form, starts in infancy
TTP
Hyaline thrombi in end arterioles and capillaries
Hyaline thrombi are composed of platelets and
von Willebrand factor with little or no fibrin or
fibrinogen
Deposition of these platelet-vWf thrombi leads to
thrombocytopenia
Degree of thrombocytopenia is related to extent
of microvascular platelet aggregation
RBCs flowing under arterial pressure fragment
when they have to manouever these thrombi in
the microvessels
TTP
Thrombotic lesions give rise to other
manifestations
Organ ischemia
Neurological
Visual
Abdominal-pain due to mesenteric ischemia, bleeding due to
thrombocytopenia
Renal
Overwhelming renal damage is not usual; if so, consider
HUS
TTP
Hemolysis can be severe
Smear shows marked decrease in platelets, RBC
polychromasia and RBC fragmentation
(microspherocytes, shistocytes) called
MICROANGIOPATHIC HEMOLYTIC ANEMIA
Coagulation tests remain normal
TTP
Accumulation of unusually large von Willebrand
factor (ULVWF)
In the plasma, ULVWF is rapidly cleaved by a
VWF cleaving metalloprotease also called “ a
disintegrin-like and metalloprotease domain with
thrombospondin type 1 motifs” (ADAMTS 13)
SO WHAT HAPPENS IN TTP?
Familial chronic relapsing TTP
Sporadic
Deficiency or absence of the Vwf cleaving protease
Autoantibody against the protease causing deficiency
or loss of function
Measurement of the vWF protease enzyme (not
rapid enough for clinical use)
THROMBOCYTOPENIA IN THE ICU
Sepsis is commonest
Often multifactorial, exact cause may be difficult to
pinpoint
Infection, sepsis, shock
Heparin
Other drugs
DIC
Massive blood transfusion
Post transfusion purpura
CPR
Cardiopulmonary bypass
ARDS
Pulmonary emboli
Intravascular catheters
DRUG INDUCED THROMBOCYTOPENIA
Drug dependent antibodies specific for the drug
structure and bind tightly to the platelets by the
Fab region in the presence of the drug
Platelets seem to be the favorite target of these
drug dependent antibodies
When should DIT be suspected?
Unexpected occurrence of thrombocytopenia
Recurrent episodes of thrombocytopenia with quick
recovery
Misdiagnosis of ITP
Beware of quinine containing agents like tonic water,
bittter lemon; foods such as tahini containing sesame
seeds, herbal remedies like Jui herbal tea
List of drugs from www.ouhsc.edu/platelets
ANTITHROMBOTIC AGENTS AND
THROMBOCYTOPENIA
Presents as acute ITP
0.1% - 2% of patients have severe
thrombocytopenia within several hours of
exposure to Abiciximab, Tirobifan or Eptifibatide
About 12% can become acutely thrombocytopenic
after second exposure to Abiciximab
Immediate reactions are due to presence of
naturally occurring antibodies against structural
elements of abiciximab or due to structural
changes to GpIIb/IIIa induced by binding of
Tirobifan and Eptifitabide.
Immune-Mediated Thrombocytopenia.
Warkentin TE. N Engl J Med 2007;356:891-893.
THROMBOCYTOPENIA
Dysplastic megakaryocytes
Myelodysplastic syndromes
Chemotherapy effects
Failure of function of megakaryocytes due to
defects in DNA synthesis
B12 deficiency
Folate deficiency
DYSPLASTIC MEGAKARYOCYTE
DYSPLASTIC MEGAKARYOCYTE
APPROACH TO THROMBOCYTOPENIA
Plt <150
Hb and
WBC
count
Normal
Abnormal
Smear
Bone
marrow
exam
Fragmente
d red cells
Normal
RBC,
platelets
normal
DIC/TTP
Consider
Drugs,
Infection,
ITP,
Congenital
THROMBOCYTOSIS
Reactive thrombocytosis
Associated with blood loss and surgery
Post splenectomy
Iron deficiency anemia
Inflammation and disease
Stress or exercise
Clonal thrombocytosis
Polycythemia vera
CML
Myelofibrosis
Primary or Essential thrombocythemia
MDS associated
THROMBOCYTOSIS IN CML
MICROMEGAKARYOCYTES IN PERIPHERAL
BLOOD
PLATELET THERAPEUTICS
Platelet transfusions
Platelet pheresis
Manipulation of the immune system
Prevention of complications
Reduction of platelet number
Hydrea
Suppression of megakaryocyte platelet production
IVIG, Steroids, Rituxan, Splenectomy, immunosuppressives
Anagrelide
Stimulation of megakaryocyte production
Thrombopoeitin mimetics or TPO mimetics
Romiplostim
Eltromobag
Inhibitors of platelet aggregation
Aspirin, Clopidrogel, NSAIDs
Gp IIb/IIIa inhibitors
Dipyridamole
THROMBOPOEITIN MIMETICS
Romiplostim
Trade name is Nplate
TPO receptor agonist
Route: subcutaneous
Mechanism: Like
endogenous TPOincreases platelet
production by binding
and activating TPO
receptor
Indications: Chronic ITP
Dose titration based on
platelet count
Eltromobag
Trade name is Promacta
TPO receptor agonist
Route: oral
Mechanism: similar to
Nplate
Indications: Chronic ITP
Dose titration based on
platelet count