Optics: Lecture 2 Light in Matter:... Dispersive Prism: Dependence of the index of refraction, n(),...
advertisement

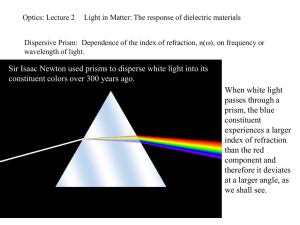
Optics: Lecture 2 Light in Matter: The response of dielectric materials Dispersive Prism: Dependence of the index of refraction, n(), on frequency or wavelength of light. Sir Isaac Newton used prisms to disperse white light into its constituent colors over 300 years ago. When white light passes through a prism, the blue constituent experiences a larger index of refraction than the red component and therefore it deviates at a larger angle, as we shall see. The effect of introducing a homogenous, isotropic dielectric changes Maxwell’s equations to the extent that o and o . The phase speed in the medium becomes: v 1 / The ratio of the speed of an E-M wave in vacuum to that in matter is defined as the index of refraction n: c n KE KM v o o KE o KM o Relative Permittivity and Relative Permeability For most dielectrics of interest that are transparent in the visible, these are essentially non-magnetic and to a good approximation KM 1. To a good approximation also known as Maxwell’s Relation: n KE KE is presumed to be the static dielectric constant (and works well only for some simple gases, as shown on next slide). In reality, KE and n are actually frequency-dependent, n(), known as dispersion. Resonant process h E n Scattering and Absorption: h m n •Non-resonant scattering: •Energy is lower than the resonant frequencies. •E-M field drives the electron cloud into oscillation •The oscillating cloud relative to the positive nucleus creates an oscillating dipole that will re-radiate at the same frequency. Em Gas/Solids Excitation energy can be transferred via collisions before a photon is re-emitted. Works well Doesn’t work so well A small displacement x from equilibrium causes a restoring force F. F = -kEx and results in resonant frequency: o k E / me E(t) x-axis + - Light E(t) and produces a classical forced oscillator. Amplitude ~ 10-17 m for bright sun light. The result can be modeled like a classical forced oscillator with FE = qeEocos(t) = qeE(t). Using Newton’s 2nd law: Driving Force – Restoring Force = ma, where Rest. Force = -kEx 2 d x 2 qe Eo cos t meo x me 2 dt To solve, let x(t) = xocost qe Eo cos t meo xo cos t me 2 xo cos t 2 qe Eo qe Eo qe E (t ) xo ; x cos t ; x 2 2 2 2 2 me o me o me o 2 *Note that the phase of the displacement x depends on > o or < o which gives x qE(t). The electric polarization or density of dipole moments (dipole moment/vol.) is given by: qe2 NE / me P qe xN 2 o 2 Where N = number electrons per volume. We learn from the dielectric properties of solids that ( o ) E P. 2 q P ( t ) Therefore e N / me o o 2 E (t ) o 2 Since n2 = KE = /o it follows that we obtain the following dispersion equation: *Note that > o n < 1 (above resonance) 2 1 (Displacement is 180 out-of-phase with q 2 e N 2 n ( ) 1 2 driving force.) o me o and < o n >1 (below resonance) (Displacement is in-phase with driving force.) Consider classically the average power (Pav) delivered by the applied electric field: q E dx dx F (t ) qe E (t ) e 0 2 dt dt me 2 02 4 2 2 2 Pav 2 (See, e.g. Mechanics, Symon, 3rd Ed.) 02 2 tan 2 Phase angle This average power is analogous to absorption of E-M radiation at the resonant frequency 0. For light = ck = 2c/, we can write the dispersion relation as (n 2 1) 1 C / 2 C / 2o ; C 4 2 c 2 o me /( Nqe2 ) Thus, if we plot (n2-1)-1 versus -2 we should arrive at a straight line. •In reality, there are several transitions in which n > 1 and n < 1 for increasing , i.e., there are several oi resonant frequencies corresponding to the complexity of the material. •Therefore, we generalize the above result for N molecules/vol. with fj different oscillators having natural frequencies oj, where j = 1, 2, 3.. 2 qe N 2 n ( ) 1 o me fj j 2 2 oj A quantum mechanical treatment shows further that f j 1 j where fj are weighting factors known as Q.M. oscillator strengths and represent the transition probability for each mode j. The energy oj is the energy of absorption or emission for a given electronic, atomic, or molecular transition. •When = oj then n is discontinuous (and blows up). Actual observations show continuity and finite n. •The conclusion is that a damping force which is proportional to the speed medx/dt should generally be included when there are strong interactions occurring between atoms and molecules, such as in liquids and solids. •With damping, (1) energy is lost when oscillators re-radiate and (2) heat is generated as a result of friction between neighboring atoms and molecules. •The corrected dispersion, including damping effects, is as follows: 2 q N n 2 ( ) 1 e o me fj j 2 2 i j oj This expression often works fine for gases. •In a dense solid material, the atoms/molecules may experience an additional field that is induced by the surrounding medium and is given by P(t)/3o. •With this induced field, the dispersion relation becomes Nqe2 n2 1 2 n 2 3 o me j fj 2 oj 2 i j •We will see that a complex index of refraction will lead to absorption. •Presently, we will consider regions of negligible absorption in which n is real and oj2 2 j. •Thus Nqe2 n2 1 2 n 2 3 o me For various glasses, j fj 2 oj 2 oj2 2 , n2 () Const. Since oj ~ 100 nm in the ultra-violet (UV). •Note that as oj, n() gradually increases and the behavior is called “Normal Dispersion.” •Again, at = oj, n is complex and leads to an absorption band. •Also, when dn/d < 0, the behavior is called “Anomalous Dispersion.” When white light passes through a glass prism, the blue constituent experiences a larger index of refraction than the red component and therefore it deviates at a larger angle, as seen in the first slide. •Note the rise of n in the UV and the fall of n in the IR, consistent with “Normal Dispersion.” •At even lower frequencies in the radio range, the materials become again transparent with n > ~1. •Transparency occurs when << o or >> o. •When ~ o, dissipation, friction and therefore absorption occurs, causing the observed opacity. Absorption Spectrum of Water: Absorption coefficient: nI is the imaginary part of the index of refraction. 2nI / c The water vapor absorption bands are related to molecular vibrations involving different combinations of the water molecule's three fundamental vibrational transitions: V1: symmetric stretch mode V2: bending mode V3: asymmetric stretch mode The absorption feature centered near 970 nm is attributed to a 2V1 + V3 combination, the one near 1200 nm to a V1 + V2 + V3 combination, the one near 1450 nm to a V1 + V3 combination, and the one near 1950 nm to a V2 + V3 combination. In liquid water, rotations tend to be restricted by hydrogen bonds, leading to vibrations, or rocking motions. Also stretching is shifted to a lower frequency while the bending frequency increased by hydrogen bonding. Propagation of Light, Fermat’s Principle (1657) Involves the principle of least time: The path between two points that is taken by a beam of light is the one that is transversed in the least amount of time. t SP SO OP t vi vt b 2 (a x) 2 h2 x2 vi vt To find the path of least time, set dt/dx=0. dt x (a x) dx vi h 2 x 2 vt b 2 (a x) 2 0 sin i sin t ni Sin i nt Sin t vi vt Since ni = c/vi and nt = c/vt . Snell’s Law of Refraction c 1 c o where o is the Note that v n n n vacuum wavelength. In general, for many layers having different n, we can write t SP m sm s s1 s2 t ... i v1 v2 vm i 1 vi 1 m c t ni si ; ni c i 1 vi Note that if the layers are very thin, we can write m n s i 1 i i P n( s)ds OPL S = OPL (Optical Path Length) We can compute t as simply OPL t . c P m Note that the spatial path length is s ds i 1 i S and for a medium possessing a fixed index n1, OPL o s . •Fermat’s principle can be re-stated: Light in going from SP traverses the route having the smallest OPL. •We will begin next with the E-M approach to light waves incident at an interface and derive the Fresnel Equations describing transmission and reflection.