Document 15523767
advertisement
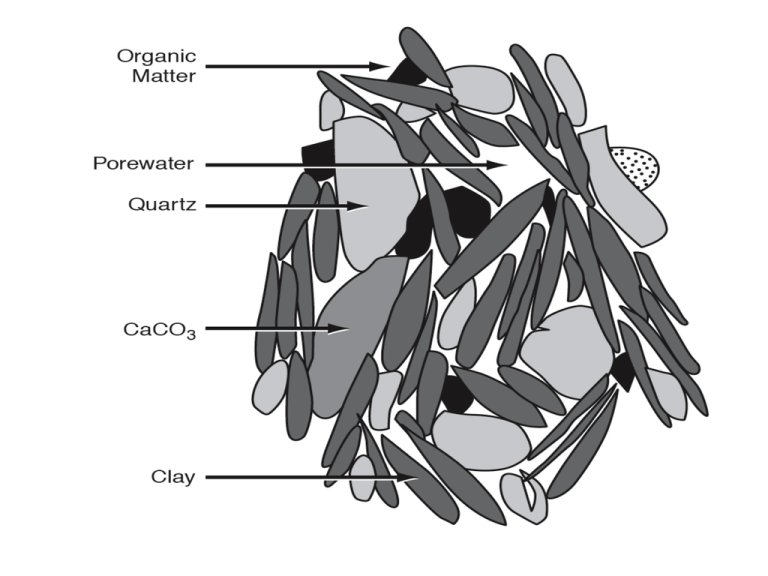
Table 6. 1.1 Organi c matter remineralization reactions based on organic matte r OM with the composition OM = C106 H175 O42N16 P. Reaction Stoichiometry (1) Aerobi c respiration OM + 150 O2 106 CO2 + 16 HNO3 + H3PO4 + 78 H2O (2) Denit rification OM + 104 HNO3 106 CO2 + 60 N2 + H3PO4 + 138 H2O (3) Manganese reduction OM + 260 MnO 2 + 174 H2O 106 CO2 + 8 N2 + H3PO4 + 260 Mn(OH) 2 (4) Iron reduction OM + 236 Fe2O3 + 410 H2O 106 CO2 + 16 NH3 + H3PO4 + 472 Fe(OH)2 (5) Sulfate reduction OM + 59 H2SO4 106 CO2 + 16 NH3 + H3PO4 + 59 H2S + 62 H2O (6) Methane fermentation (methanogenesis) OM + 56 H2O 47 CO2 + 59 CH4 + 16 NH3 + H3PO4 Table 6. 1.2 Free energy changes fo r remineralizat ion reactions (Morel and Hering [1993]). CH2O represents sucrose. Reaction Free energy change (kJ mol –1 of CH2O) -476 CH2O + O2 CO2 + H2O 5CH2O + 4 NO3- 2 N2 + 4 HCO3- + CO2+ 3 H2O -452 CH2O + 3 CO2 + H2O + 2 MnO 2 2 Mn++ + 4 HCO3- -388 CH2O + 7 CO2 + 4 Fe(OH) 3 4 Fe++ + 8 HCO3- + 3 H2O -187 2 CH2O + SO4-- H2S + 2HCO3- -82 2CH2O CO2 + CH4 -71 Table 6.1.3 Indirect oxidation reactions based on organic matter OM with the composition OM = C106H175 O42 N16P proposed by Anderson [1995]. The top three rows depict the coupli ng of m ethane fermentation with anaerobic oxidation of methane by sulf ate to produce an overall reaction with the stoichiometry of the sulf ate reduction reaction. The lower three rows depict the coupli ng o f sulf ate reduction with the reaction of sulfi de and amm onium with oxygen to give an overall reaction that has the same stoichiometry as aerobic respiration. Reaction Stoichiometry OM + 56 H2O Methane fermentation 47 CO2 + 59 CH4 + 16 NH3 + H3PO 4 59 H2SO4 + 59 CH4 Anaerobic oxidation of methane 59 CO2 + 118 H2 O + 59 H2S OM + 59 H2SO4 Overall reaction 106 CO2 + 16 NH3 + H3 PO4 + 59 H2S + 62 H2O OM + 59 H2SO4 Sulfa te reduction 106 CO2 + 16 NH3 + H3 PO4 + 59 H2S + 62 H2O Oxidation of sulfide and 59 H2S + 16 NH3 + 150 O2 ammonium 59 H2SO4 + 16 HNO 3 + 16 H2 O OM + 150 O2 Overall reaction 106 CO2 + 16 HNO 3 + H3 PO4 + 78 H2 O Table 6.1.4 Contribution of various sedim ent reminerali zation processes to global oxidation rate. Estimate of global organic carbon oxidation rate Remineralization Reaction % of Total Pg C yrΠ1 65.0% 1.6 Aerobic respiration 6.5% 0.16 ± 0.05 Denitrification ~0.6% 0.01 Π0.02 Manganese Reduction ~0.3% 0.003 Π0.010 Iron Reduction 17.9% 0.44 Sulfa te Reduction 9.8% 0.24 Methanogenesis 2.5 TOTAL Table 6.2.2 Diagenetic equations for a dissolved constituent C in pore water, and a soli d constituent B in the soli d sedim ents. C has units of mmol mΠ3 of pore water, and B has units of mmol mΠ3 of dry soli ds. C C B C B,C w C s 1 r R C:B max t z z z K B +B K C +C 1 B B B C B,C 1 S B 1 DB 1 R max t z z z K +B B K C +C Table 6.3.1 The relative contribution of various oxidants to organic matter remi nerali zation at representative sites. Site Percent of Total NO 3 SO 2O2 Mn & Fe 4 Coastal sediments Danish coast 40 3 57 Cape Lookout Bight 68 Saanich Inlet 76 Continental Slope and Rise (<1500 m) Central Calif ornia (3 cores) 29 57 0.8 14 a Washington State (19 cores) 10 35 55 a NW Mexico (19 cores) 0 45 55 (³1500 m) Central Calif ornia (5 cores) 71 17 1.8 10 a Washington State (4 cores) 62 26 12 a NW Mexico (5 cores) 60 30 9 (Other) Indian Ocean 87 12 0.3 0.6 Hatteras Cont. Rise 76 8 1.7 14 Bermuda Rise 78 12 1.4 9 Savu Basin 61 25 6.5 7.2 Deep Sea Manop H 99 0.8 0.4 Manop M 91 6.9 0.4 Hatteras Abyssal Plain 96 4 - CH4 32 24 - Table 6. 5.1 POC budge t (Pg C yr–1). The % is with respect to the global total for each row. Deep Ocean Continental margin Global (>1000 m) (<1000 m) Ocean area (x10 14 km2) 3.221 (92%) 0.276 (8%) 3.497 Water Column POC Budget a Production 45 ± 7 (84%) 8.6 ± 0.8 (16%) 53 ± 8 b Export (100 m) 7.0 ± 2.4 (71%) 2.8 ± 0.9 (29%) 9.8 ± 3.3 c Remineralization 6.6 ± 2.4 (91%) 0.63 ± 1.13 (9%) 7.3 ± 3.4 Flux to seafloor 0.34 ± 0.14 (14%) 2.2 ± 0.7 (86%) 2.5 ± 0.8 Sediment POC Budget e Remineralization 0.24 ± 0.14 (11%) 1.9 ± 0.7 (89%) 2.1 ± 1.3 f Burial 0.018 ± 0.016 (9%) 0.174 ± 0.066 (91%) 0.19 ± 0.07 Other Sediment Processes g DOC flux t o water column 0.080 ± 0.033 (40%) 0.120 ± 0.039 (60%) 0.20 ± 0.07 h Denitrification 0.032 ± 0.014 (19%) 0.133 ± 0.043 (81%) 0.165 ± 0.054 Table 6. 5.1 POC budge t. The % is with respect to the global total fo r each row. Deep Ocean Continental margin Global (>1000 m) (<1000 m) Ocean area (x10 14 km2) 92% 8% 3.497 Water Column POC Budget Production a 84% 16% 53 ± 8 Export (100 m)b 71% 29% 9.8 ± 3.3 Remineralization c 91% 9% 7.3 ± 3.4 Flux to seafloor 14% 86% 2.5 ± 0.8 Sediment POC Budget Remineralization e 11% 89% 2.1 ± 1.3 Burial f 9% 91% 0.19 ± 0.07 Other Sediment Processes DOC flux t o water column g 40% 60% 0.20 ± 0.07 Denitrification h 19% 81% 0.165 ± 0.054 Diagenetic Equations Pore water: C ADV(C) DIFF(C) SMS(C) t Solid particles: 1 B ADV (B) DIFF(B) SMS(B) t (6.2.1a) (6.2.1a) Diffusion in pore water - 1 Fick’s First Law C (z) s z C (6.2.2) Fick's Second Law (z) C DIFF(C) s (6.2.3) z z z C Diffusion in pore water - 2 Start with C (z) e z C (1) NOTE: There is considerable confusion in the literature resulting from the failure to differentiate clearly between the tortuosity factor and the tortuosity . Advection in pore water ADV (C) wPW C z (6.2.4) (1) Full equation Remineralization in Sediments B C B,C SMS(C) 1 rC:B Rmax K B +B K C +C (2) For C » KC, (e.g., KC,~3 mmol m –3 for O2) B B SMS(C) 1 rC :B Rmax (1) K B +B (3) For KB » B (ofte n used for aerobic respiratio n) SMS(C) 1 k B (2a) B Rmax k rC:B . (2b) KB (4) For C < KC, but where KB » B (generally used for denitrification , where KB ~ 20 mmo l m-3) C SMS(C) 1 k B (3a) K C +C B,C Rmax k rC:B , KB (5) G-type kinetics (3b) n GT Gi (4) i1 n SMS(GT ) (1 ) ki Gi i1 (5) (6.2.5) Diffusion and advection of solid particles B DIFF(B) 1 DB z z ADV (B) 1 S B z (6.2.9) (6.2.10) Bioturbated layer thickness from radiocarbon Bioturbation of 210Pb (1) Full equation [ 210 Pb] 210 0 1 D 1 [ Pb] B Pb-210 z z = APb-210 1 D Pb-210 1 APb-210 B z z (6.2.15) (2) Assume porosity an d bioturbatio n are constant with depth 2 APb-210 (6.2.16) 0 DB Pb-210 APb-210 2 z (3) boundar y conditions APb-210 = (APb-210)0 at z = 0, and APb-210 0 as z z APb-210 (APb-210 )0 exp z* (6.2.17) DB z* POC Equation O2 Equation