MECH 558 Combustion Fall 2015
advertisement

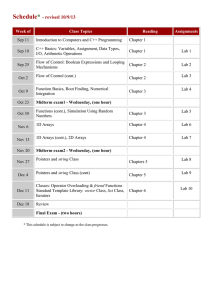
MECH 558 Combustion Syllabus Fall 2015 Catalog Description: Combustion processes: explosions, detonations, flame propagation, ignition, generation of pollutants in moving and stationary energy conversion systems. Textbook: An Introduction to Combustion: Concepts and Applications, Stephen R. Turns, Third Edition, McGraw Hill, 2011. Instructor: Anthony J. Marchese High speed OH laser induced fluorescence of a Office: ENG A103H turbulent inverse diffusion flame. Phone: (970) 491-2328 Email: marchese@colostate.edu Office Hours: M, W 11:00 a.m. to 12:00 p.m. (or email me to make an appointment) Technical Objectives: Combustion refers to the study of chemically reacting fluid systems. Thus, this course utilizes all of the fundamental tools acquired during the study of Chemistry, Thermodynamics, Heat Transfer, and Fluid Mechanics. Upon completion of this course, each student will be able to: Calculate adiabatic flame temperature and combustion products using the concepts of chemical equilibrium. Derive the Arrhenius rate expression from simple kinetic theory. Explain the explosion limits of the H2/O2 system using chemical kinetic arguments. Explain qualitatively the chemical oxidation mechanism of alcohols, alkanes and aromatics, and explain the NTC region in alkane oxidation via elementary chemical kinetic arguments. Assemble detailed chemical kinetic mechanisms and model zero and one-dimensional chemically reacting systems using CHEMKIN software. Calculate premixed laminar flame speed using phenomenological arguments and perform complex laminar flame calculations using CHEMKIN software. Determine the burning rate of a liquid fuel droplet. Explain the formation mechanisms of NOx, HC, CO and PM in combustion systems and how to design systems with low pollutant formation. Grading Policy: Homework and Class Participation (35%). Homework will be assigned on a periodic basis. Homework assignments will be in the form of problems or short projects. Midterm Exam (30%). A written midterm exam will be given approximately half-way through the semester Final Exam (35%). A comprehensive oral final exam will be given during the final exam week. Attendance Policy and Rules Attendance at each class is required. Class participation is a key component of the course. Notebooks and calculators should be brought to each class. Cell phones must be turned off, unless you have an emergency situation then please set to vibrate. Information Information on this class (including this syllabus) will be posted in the web at: http://www.engr.colostate.edu/~marchese/combustion11 Course Outline Week Text Ch. 1 Ch. 2 Topics Course Overview; Combustion Terminology and Gas Mixtures Ch. 2 Chemical equilibrium; Calculation of adiabatic flame temperature. Ch. 2 Chemical equilibrium; Calculation of adiabatic flame temperature using NASA CEA Code Sep. 14 Sep. 16 Sep. 18 Sep. 21 Sep. 23 Sep. 25 Ch. 3 Mass Transfer Ch. 4 Introduction to Chemical kinetics. Global vs. elementary reactions. Derivation of Arrhenius rate expression from kinetic theory. Chain reactions. 6 Sep. 28 Sep. 30 Oct. 2 Ch. 5 Hydrogen- Oxygen explosion limits. Chain branching. Carbon Monoxide Explosion Limits. 7 Oct. 5 Oct. 7 Oct. 9 Ch. 5 App. 2A Nomenclature in Organic Chemistry Oct. 12 Oct. 14 Oct. 16 Ch. 6 /Ch. 7 Coupling Chemical and Thermal Analysis of Reacting Systems 1 2 3 4 5 8 Date(s) Aug. 24 Aug. 26 Aug. 28 Aug. 31 Sep. 2 Sep. 4 Sep. 7 – no class Sep. 9 Sep. 11 Homework Oxidation characteristics of hydrocarbons: alcohols, alkanes, aromatics. CHEMKIN transient homogeneous H2/O2 calculations 9 10 11 12 13 Oct. 19 Oct. 21 Oct. 23 Oct. 26 Oct. 28 Oct. 30 Nov. 2 Nov. 4 Nov. 6 Nov. 9 Nov. 11 Nov. 13 Nov. 16 Nov. 18 Nov. 20 14 Nov. 23 - 27 15 Nov. 30 Dec. 2 Dec. 4 Dec. 7 Dec. 9 Dec. 11 16 17 Dec. 15-19 Notes CHEMKIN plug flow reactor and engine calculations Ch. 8 Premixed Laminar Flames Ch. 8 Premixed Laminar Flames CHEMKIN Modeling of Premixed Laminar Flames Ch. 9 Laminar Diffusion Flames Fall Break Ch. 10 Droplet Evaporation and Combustion Ch. 15 Environmental Considerations: Smog, Oxides of Nitrogen (NOx), Particulate Matter Oral Final Exams Midterm, Date TBA