Crystal Structure Continued!
advertisement

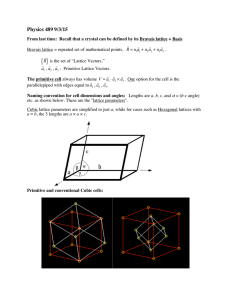
Crystal Structure Continued! • NOTE!! Much of the discussion & many figures in what follows were constructed from lectures posted on the web by Prof. Beşire GÖNÜL in Turkey. She has done an excellent job of covering many details of crystallography & she illustrates her topics with many very nice pictures of lattice structures. Her lectures on this are posted Here: http://www1.gantep.edu.tr/~bgonul/dersnotlari/. Her homepage is Here: http://www1.gantep.edu.tr/~bgonul/. Crystal Lattices Bravais Lattices Non-Bravais Lattices (BL) (non-BL) All atoms are the same kind All lattice points are equivalent Atoms are of different kinds. Some lattice points aren’t equivalent. Atoms are of different kinds. Some A combination 2 or more BL lattice points areofnot equivalent. 2 d examples Lattice Translation Vectors In General • Mathematically, a lattice is defined by 3 vectors called Primitive Lattice Vectors a1, a2, a3 are 3d vectors which depend on the geometry. • Once a1, a2, a3 are specified, the Primitive Lattice Structure is known. • The infinite lattice is generated by translating through a Direct Lattice Vector: T = n1a1 + n2a2 + n3a3 n1,n2,n3 are integers. T generates the lattice points. Each lattice point corresponds to a set of integers (n1,n2,n3). 2 Dimensional Lattice Translation Vectors Consider a 2-dimensional lattice (figure). Define the 2 Dimensional Translation Vector: Rn n1a + n2b (Sorry for the notation change!!) a & b are 2 d Primitive Lattice Vectors, n1, n2 are integers. Point D(n1, n2) = (0,2) Point F(n1, n2) = (0,-1) • Once a & b are specified by the lattice geometry & an origin is chosen, all symmetrically equivalent points in the lattice are determined by the translation vector Rn. That is, the lattice has translational symmetry. Note that the choice of Primitive Lattice vectors is not unique! So, one could equally well take vectors a & b' as primitive lattice vectors. The Basis (or basis set) The set of atoms which, when placed at each lattice point, generates the Crystal Structure. Crystal Structure ≡ Primitive Lattice + Basis Translate the basis through all possible lattice vectors T = n1a1 + n2a2 + n3a3 to get the Crystal Structure or the Direct Lattice • The periodic lattice symmetry is such that the atomic arrangement looks the same from an arbitrary vector position r as when viewed from the point r' = r + T (1) where T is the translation vector for the lattice: T = n1a1 + n2a2 + n3a3 • Mathematically, the lattice & the vectors a1,a2,a3 are Primitive if any 2 points r & r' always satisfy (1) with a suitable choice of integers n1,n2,n3. • In 3 dimensions, no 2 of the 3 primitive lattice vectors a1,a2,a3 can be along the same line. But, DO NOT think of a1,a2,a3 as a mutually orthogonal set! Often, they are neither mutually perpendicular nor all the same length! • For examples, see Fig. 3a (2 dimensions): The Primitive Lattice Vectors a1,a2,a3 aren’t necessarily a mutually orthogonal set! Often, they are neither mutually perpendicular nor all the same length! • For examples, see Fig. 3b (3 dimensions): Crystal Lattice Types Bravais Lattice An infinite array of discrete points with an arrangement & orientation that appears exactly the same, from whichever of the points the array is viewed. A Bravais Lattice is invariant under a translation T = n1a1 + n2a2 + n3a3 Nb film Non-Bravais Lattices •In a Bravais Lattice, not only the atomic arrangement but also the orientations must appear exactly the same from every lattice point. 2 Dimensional Honeycomb Lattice • The red dots each have a neighbor to the immediate left. The blue dot has a neighbor to its right. The red (& blue) sides are equivalent & have the same appearance. But, the red & blue dots are not equivalent. If the blue side is rotated through 180º the lattice is invariant. The Honeycomb Lattice is NOT a Bravais Lattice!! Honeycomb Lattice It can be shown that, in 2 Dimensions, there are Five (5) & ONLY Five Bravais Lattices! 2-Dimensional Unit Cells Unit Cell The Smallest Component of the crystal (group of atoms, ions or molecules), which, when stacked together with pure translational repetition, reproduces the whole crystal. b S a S S S S S S S S S S S S S S Unit Cell The Smallest Component of the crystal (group of atoms, ions or molecules), which, when stacked together with pure translational repetition, reproduces the whole crystal. Note that the choice of unit cell is not unique! S S S 2-Dimensional Unit Cells – Artificial Example: “NaCl” Lattice points are points with identical environments. 2-Dimensional Unit Cells: “NaCl” Note that the choice of origin is arbitrary! the lattice points need not be atoms, but The unit cell size must always be the same. 2-Dimensional Unit Cells: “NaCl” These are also unit cells! It doesn’t matter if the origin is at Na or Cl! 2-Dimensional Unit Cells: “NaCl” These are also unit cells. The origin does not have to be on an atom! 2-Dimensional Unit Cells: “NaCl” These are NOT unit cells! Empty space is not allowed! 2-Dimensional Unit Cells: “NaCl” In 2 dimensions, these are unit cells. In 3 dimensions, they would not be. 2-Dimensional Unit Cells Why can't the blue triangle be a unit cell? Example: 2 Dimensional, Periodic Art! A Painting by Dutch Artist Maurits Cornelis Escher (1898-1972) Escher was famous for his so called “impossible structures”, such as Ascending & Descending, Relativity,.. Can you find the “Unit Cell” in this painting? 3-Dimensional Unit Cells 3-Dimensional Unit Cells 3-Dimensional Unit Cells 3 Common Unit Cells with Cubic Symmetry Simple Cubic (SC) Body Centered Cubic (BCC) Face Centered Cubic (FCC) Conventional & Primitive Unit Cells Unıt Cell Types Primitive Conventional (Non-primitive) A single lattice point per cell More than one lattice point per cell The smallest area in 2 dimensions, or The smallest volume in 3 dimensions Volume (area) = integer multiple of that for primitive cell Simple Cubic (SC) Conventional Cell = Primitive cell Body Centered Cubic (BCC) Conventional Cell ≠ Primitive cell Face Centered Cubic (FCC) Structure Conventional Unit Cells • A Conventional Unit Cell just fills space when translated through a subset of Bravais lattice vectors. • The conventional unit cell is larger than the primitive cell, but with the full symmetry of the Bravais lattice. • The size of the conventional cell is given by the lattice constant a. FCC Bravais Lattice The full cube is the Conventional Unit Cell for the FCC Lattice Conventional & Primitive Unit Cells Face Centered Cubic Lattice Primitive Unit Cell (Shaded) Lattice Const. Primitive Lattice Vectors a1 = (½)a(1,1,0) a2 = (½)a(0,1,1) a3 = (½)a(1,0,1) Note that the ai’s are Conventional Unit Cell (Full Cube) NOT Mutually Orthogonal! Elements That Form Solids with the FCC Structure Body Centered Cubic (BCC) Structure Conventional & Primitive Unit Cells Body Centered Cubic Lattice Primitive Lattice Primitive Unit Cell Vectors a1 = (½)a(1,1,-1) a2 = (½)a(-1,1,1) Lattice a3 = (½)a(1,-1,1) Constant Conventional Unit Cell (Full Cube) Note that the ai’s are NOT mutually orthogonal! Elements That Form Solids with the BCC Structure Conventional & Primitive Unit Cells Cubic Lattices Simple Cubic (SC) c b Primitive Cell = Conventional Cell Fractional coordinates of lattice points: 000, 100, 010, 001, 110,101, 011, 111 a b c Body Centered Cubic (BCC) Primitive Cell Conventional Cell a b c Fractional coordinates of the lattice points in the conventional cell: 000,100, 010, 001, 110,101, 011, 111, ½ ½ ½ a Primitive Cell = Rombohedron Conventional & Primitive Unit Cells Cubic Lattices Face Centered Cubic (FCC) Primitive Cell Conventional Cell The fractional coordinates of lattice points in the conventional cell are: 000,100, 010, 001, 110,101, 011, 111, ½ ½ 0, ½ 0 ½, 0 ½ ½, ½ 1 ½, 1 ½ ½ , ½ ½ 1 b c a Simple Hexagonal Bravais Lattice Conventional & Primitive Unit Cells Points of the Primitive Cell Hexagonal Bravais Lattice Primitive Cell = Conventional Cell c b a Fractional coordinates of lattice points in conventional cell: 100, 010, 110, 101, 011, 111, 000, 001 Hexagonal Close Packed (HCP) Lattice: A Simple Hexagonal Bravais Lattice with a 2 Atom Basis The HCP lattice is not a Bravais lattice, because the orientation of the environment of a point varies from layer to layer along the c-axis. General Unit Cell Discussion • For any lattice, the unit cell &, thus, the entire lattice, is UNIQUELY determined by 6 constants (figure): a, b, c, α, β and γ which depend on lattice geometry. • As we’ll see, we sometimes want to calculate the number of atoms in a unit cell. To do this, imagine stacking hard spheres centered at each lattice point & just touching each neighboring sphere. Then, for the cubic lattices, only 1/8 of each lattice point in a unit cell is assigned to that cell. In the cubic lattice in the figure, Each unit cell is associated with (8) (1/8) = 1 lattice point. Primitive Unit Cells & Primitive Lattice Vectors • In general, a Primitive Unit Cell is determined by the parallelepiped formed by the Primitive Vectors a1 ,a2, & a3 such that there is no cell of smaller volume that can • The Primitive Unit Cell volume can be found by • As we’ve discussed, a Primitive vector manipulation: V = a1(a2 a3) Unit Cell can be repeated to fill space by periodic repetition of it • For the cubic unit cell in through the translation vectors the figure, V = a3 be used as a building block for the crystal structure. T = n1a1 + n2a2 + n3a3. Primitive Unit Cells • Note that, by definition, the Primitive Unit Cell must contain ONLY ONE lattice point. • There can be different choices for the Primitive Lattice Vectors, but the Primitive Cell volume must be independent of that choice. 2 Dimensional Example! j P = Primitive Unit Cell NP = Non-Primitive Unit Cell