Influence_storage_time.doc
advertisement

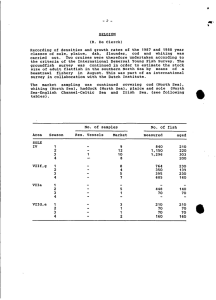
1 2 INFLUENCE OF STORAGE TIME AND TEMPERATURE ON LIPID 3 DETERIORATION DURING COD (Gadus morhua) AND HADDOCK 4 Melanogrammus aeglefinus) FROZEN STORAGE 5 6 7 8 9 10 Santiago P. Aubourg* and Isabel Medina 11 12 Instituto de Investigaciones Marinas (CSIC) 13 c/ Eduardo Cabello, 6 14 36208-Vigo (Spain) 15 16 17 18 19 20 21 *Author to whom correspondence should be addressed 22 [telephone +34 986 231930; fax +34 986 292762; e-mail: saubourg@iim.csic.es] 23 24 ABSTRACT 1 2 3 Lean fish deterioration during frozen storage (-30C and -10C) up to one year 4 was studied by lipid changes assessment. Comparison between a formaldehyde (FA)- 5 forming (cod) and a FA-non forming (haddock) species was carried out. Lipid damages 6 were measured on the basis of free fatty acids (FFA), peroxide value (PV), 7 thiobarbituric acid index (TBA-i) and fluorescent compounds. In both species, at -30ºC 8 most lipid damage indices showed significant correlations with the storage time. 9 However, at -10ºC, only the FFA and fluorescence detections provided significant 10 correlations with the storage time. Comparison between the fish species showed higher 11 lipid oxidation (PV and TBA-i) and hydrolysis (FFA content) in haddock than in cod at 12 –10ºC; however, a higher fluorescence development was observed in cod at the same 13 temperarure. At –30ºC little differences in lipid damage indices were detected between 14 both species. 15 16 17 18 Running Title: Lipid deterioration in frozen lean fish. 19 20 21 Key Words: Formaldehyde, frozen storage, gadoids, lean fish, lipid oxidation and hydrolysis. 22 2 INTRODUCTION 1 2 3 Processed fish and other marine species are products of great economic 4 importance in many countries. During processing and storage fish quality may decline 5 as a result of several factors. One of the most important concerns the oxidation of the 6 highly unsaturated lipids directly related to the production of off flavours and odours in 7 foods (Pearson et al., 1977; Pigott and Tucker, 1987). 8 During frozen storage of lean fish such as gadoid species most attention has been 9 given to the formaldehyde (FA) formation and its implication in quality loss (Shenouda, 10 1980; Rehbein, 1988). However, lipid hydrolysis and oxidation have been shown to 11 occur during the lean fish frozen storage and become an important factor of fish 12 acceptance as influencing protein denaturation, texture changes, functionality loss and 13 fluorescence development (Davies and Reece, 1982; Mackie, 1993; Sotelo et al., 1995). 14 The relative influence of FA and lipid degradation products in texture changes has been 15 evaluated (Rehbein and Orlick, 1990; Careche and Tejada, 1994) and the participation 16 of FA in the interaction compounds formation with fluorescent properties has been 17 recently reported (Aubourg, 1998a, 1998b). 18 In the present work the influence of time (up to one year) and temperature (- 19 10ºC and –30ºC) on lipid deterioration produced during the frozen storage of lean fish 20 was studied. Detection of primary and secondary lipid oxidation products, interaction 21 compounds and lipid hydrolysis were carried out. Comparison between a FA-forming 22 fish species (cod) and a FA-non forming one (haddock) (Mackie, 1993; Howell et al., 23 1996) was undergone to study the effect that FA formed during the frozen storage may 24 have on the lipid deterioration. 25 3 MATERIALS AND METHODS 1 2 3 Raw material, processing and sampling 4 Fresh cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) were 5 purchased at a public market. Individual fish were eviscerated, beheaded, filleted and 6 frozen at -40C. The fish fillets (145-160g each, in both species) were then distributed 7 into two storage temperatures: -30C and -10C. For each storage temperature and each 8 fish species, fillets were divided into three batches, which were studied separately 9 during the whole experiment. Analyses on cod and haddock fish were carried out on the 10 homogenised white muscle of the raw material employed and at 1, 3, 5, 7, 9 and 12 11 months of frozen storage. 12 13 Water and lipid contents 14 Water content was determined by weight difference of the homogenised muscle 15 (1-2 g) before and after 24 hours at 105C. Results were calculated as g water per kg 16 muscle. Lipids were extracted by the Bligh and Dyer (1959) method. Results were 17 calculated as g lipid per kg wet muscle. 18 19 Lipid damage measurements 20 Free fatty acids (FFA) content was determined by the Lowry and Tinsley (1976) 21 method based on complex formation with cupriacetate-pyridine. Results are expressed 22 as g FFA per kg lipids. 23 24 Peroxide value (PV) expressed as meq oxygen per kg lipids was determined by the ferrithiocyanate method (Chapman and McKay, 1949). 4 1 2 3 4 The thiobarbituric acid index (TBA-i) (mg malondialdehyde per kg sample) was determined according to Vyncke (1970). All spectrophotometric determinations were carried out using a Beckman DV-64 spectrophotometer. 5 Fluorescence formation (Perkin-Elmer LS 3B) at 393/463 nm and 327/415 nm 6 was studied according to previous experience (Aubourg and Medina, 1997; Aubourg et 7 al., 1998). The relative fluorescence (RF) was calculated as follows: RF = F/Fst, where F 8 is the sample fluorescence at each excitation/emission maximum, and Fst is the 9 corresponding fluorescence intensity of a quinine sulphate solution (1 g ml-1 in 0.05 M 10 H2SO4) at the corresponding wavelength. The fluorescence shift (F) was calculated as 11 the ratio between both RF values: F = RF393/463nm / RF327/415nm, and was analysed on the 12 aqueous (Faq) and organic (For) phases resulting from the lipid extraction (Bligh and 13 Dyer, 1959). 14 15 16 17 Statistical analysis Data from the different lipid damage measurements were subjected to the ANOVA one-way method and correlation analysis (p < 0.05) (Statsoft, 1994). 18 19 20 21 RESULTS 22 23 Water contents ranged between 791 and 832 g per kg in cod and between 793 24 and 821 g per kg in haddock. Lipid contents ranged between 4.5 and 6.5 g per kg, for 5 1 both cod and haddock. No significant differences (p < 0.05) were obtained in both 2 parameters as a result of the time and temperature of frozen storage. 3 4 Lipid hydrolysis (Tables 1-4) 5 A great hydrolytic activity was observed in both fish species. The preservative 6 effect of the storage temperature was evident since -30C sample values were lower 7 than the corresponding -10C ones. As a general behaviour, the FFA formation was 8 faster during the first steps of the storage. At the end of the storage time a lower 9 (p<0.05) FFA level was obtained in the case of cod than haddock at both temperatures. 10 Haddock provided a progressive increase with time of storage at both 11 temperatures, until month 7 at -30ºC and until month 9 at -10ºC; after those time 12 storages, no significant differences were observed. Cod showed at -10C an increase till 13 month 5, and then no more differences till the end; however, at -30C an increase till 14 month 7 was followed by a decrease at the end of the storage. 15 16 Lipid oxidation (Tables 1-4) 17 The PV provided a gradual increase along the whole storage in both species at - 18 30C; however, values obtained at the end of the storage (7.9 and 8.5 for cod and 19 haddock, respectively) remained relatively low (Pérez-Villarreal and Howgate, 1991; 20 Vidya Sagar Reddy et al., 1992) as a result of the preservative effect of the storage 21 temperature on the fillets. A faster PV development was obtained at -10C in both 22 species; for cod, an increase at month 3 was followed by no variations till month 9 and a 23 decrease at the end of the storage. In the case of haddock, increases were observed at 24 months 3 and 9 followed by a sharp decrease at the end of the storage. PV comparison 6 1 of both fish species during the increasing period (months 3, 5, 7 and 9) showed a 2 significantly (p<0.05) higher primary oxidation for haddock than cod at -10C. 3 Secondary lipid oxidation was studied by the TBA-i. As a general rule, little 4 significant differences were observed. Values obtained along the whole experiment 5 were relatively low, specially compared to those obtained in fatty fish species (Kurade 6 and Baranowski, 1987; Aubourg et al., 1998). 7 At -30ºC the highest values were obtained at month 9 in haddock and at the end 8 of the storage in cod. At -10ºC, both species showed the highest values at month 5, 9 being higher in haddock than cod; then, a decrease in the formation of thiobarbituric 10 acid (TBA) reactive substances was observed that could be explained as a result of 11 combining with proteins to form polymers (Orlick et al., 1991; Vidya Sagar Reddy et 12 al., 1992). 13 14 Interaction compounds formation (Tables 1-4) 15 Interaction compounds formation was studied by means of fluorescent 16 properties, according to previous research (Aubourg and Medina, 1997; Aubourg et al., 17 1998). 18 The organic phase (lipid extract) study provided very little significant 19 differences along the first steps of storage (months 1, 3 and 5) in both species at both 20 temperatures. In all cases, the highest values were obtained at month 7 and were 21 followed by a decrease at the end of the storage time. Significant differences between 22 both fish species were only obtained at month 7, with a higher For value for haddock 23 than cod. As a result of the storage temperature, higher For values at -10C than at - 24 30C were obtained in the two latest storage times (months 9 and 12). 7 1 A clearer trend was obtained by analysis of the aqueous phase (Faq) resulting 2 from the lipid extraction. No significant variations were observed during the first five 3 months of storage at both temperatures in both species. After this induction period, 4 higher values were obtained at -10C than at -30C in the two latest storage times 5 (months 9 and 12). At -10ºC a big increase was observed in both species at month 9. At 6 -30ºC some increases were also observed (month 9 for cod and month 7 for haddock). 7 Comparison of both fish species at -10C showed that a higher Faq value was 8 produced in cod than in haddock from month 5 till the end. Little differences between 9 both species were obtained at -30C. 10 11 12 13 Correlation analyses The different quality measurements were tested for correlation with storage time and also with each other (Tables 5-6, cod; Tables 7-8, haddock). 14 According to results at –30ºC (Tables 1 and 3), all indices except for For 15 showed significant (p<0.05) linear correlation values with the storage time (Tables 5 16 and 7). As lipid degradation increased (-10ºC samples; Tables 2 and 4), some indices 17 (PV and TBA-i) showed lower correlation values with the storage time; the best linear 18 correlations were then obtained for the FFA and Faq values (Tables 6 and 8). 19 Values obtained for FFA and Faq at both temperatures and in both fish species 20 were also studied by nonlinear fittings. In most cases, the nonlinear model was better 21 than the linear one (exponential for Faq and logarithmic for FFA; Tables 5-8), 22 according to the slopes showed in Figures 1 (cod) and 2 (haddock). 23 Comparison of the different lipid damage indices between each other provided 24 some significant correlation values, although most results were not satisfactory. It can 25 be argued that the three lipid oxidation indices (PV, TBA-i and Faq) assess damage at 8 1 different steps of the whole oxidation mechanism; while hydrolysis (FFA formation) 2 follows a different pathway than oxidation. FFA and Faq indices showed, however, 3 significant correlation values at both temperatures and in both species. 4 5 6 7 DISCUSSION 8 9 The preservative effect of temperature on lipid damage was evident in both fish 10 species. Lipid hydrolysis (FFA content) and oxidation (PV and TBA-i) and interaction 11 compound formation (Faq) showed a higher development at -10ºC than at -30ºC. 12 Both the FFA content and Faq value have shown satisfactory correlation values 13 with the storage time at both temperatures tested. Reliability of the remaining indices 14 showed to decrease when considering the -10ºC temperature. As an explanation, 15 degradation products that are susceptible to be measured in such indices (peroxides, 16 TBA reactive substances) can either be destroyed or interact with other constituents, so 17 that the determination cannot always afford an accurate method for the quality changes 18 assessment (Melton, 1983; Smith et al., 1990). Thus, correlation values of the different 19 indices between each other did not provide satisfactory results. 20 According to the general theory, lipid oxidation compounds have reacted with 21 nucleophilic biological constituents (Pokorný, 1977; Gardner, 1979; Howell, 1995) and 22 caused the formation of interaction compounds with fluorescent properties. Its detection 23 by the Faq value has provided a good assessment of quality changes, according to 24 previous research where fatty fish processing (frozen storage, chilling and canning) was 25 tested (Aubourg and Medina, 1997; Aubourg et al., 1998). 9 1 At the same time, hydrolytic activity also showed to be sensitive with the time of 2 storage at both temperatures. Previous experiments on frozen storage of lean fish had 3 already shown this kind of damage determination as a valuable tool in order to assess 4 quality (Quaranta and Pérez, 1983; de Koning and Mol, 1991). 5 Formation of FFA itself does not lead to nutritional losses. However, it has been 6 proved that accumulation of FFA in frozen fish is related in some extent with lack of 7 acceptability of frozen fish, because FFA are known to cause texture deterioration by 8 interacting with proteins (Shenouda, 1980; Rehbein, 1988; Sotelo et al., 1995) and have 9 shown to be strongly interrelated with lipid oxidation (Miyashita and Takagi, 1986; Han 10 and Liston, 1988). 11 Another aspect studied in the present experiment was the comparison of lipid 12 damages between a FA-forming species (cod) and a FA-non forming (haddock) one. In 13 a previous experiment (Howell et al., 1996), cod and haddock fillets were stored at – 14 20ºC and –30ºC. As a result, formation of dimethylamine (DMA) and FA was only 15 confirmed at –20ºC in cod and not in haddock; at –30ºC neither of both fish species 16 produced DMA nor FA, according to other experiment (Pérez-Villarreal and Howgate, 17 1991). In the present work, little differences in the lipid oxidation and hydrolysis 18 development between both species were obtained at –30ºC. However, at –10ºC, when 19 FA is suposed to be produced in cod (Howell et al., 1996), a higher lipid oxidation (PV 20 and TBA-i) and hydrolysis (FFA) development was observed in haddock than in cod. 21 Result on a previous report at –10ºC concerning dehydrated samples showed that 22 haddock produced a higher pentenal content and PV than cod (Hardy and McGill, 23 1990). 24 Previous results have suggested an inhibition of FA and DMA formation due to 25 the presence of oxidised lipid in a FA-forming species (hake) during frozen storage 10 1 (Careche and Tejada, 1990; Joly et al., 1997). However, no information is available 2 related to the effect of FA on enzymes responsible for lipid hydrolysis (lipases, 3 phospholipases) and oxidation (lipoxygenases, oxidases), although FA has shown to 4 react easily with proteins and led to protein denaturation in FA-forming fish (Rehbein, 5 1988; Mackie, 1993). 6 Higher Faq values were obtained in the case of cod than haddock at –10ºC. This 7 result agrees with previous experiments where it was concluded that the presence of FA 8 would enhance the fluorescent compounds formation by participating in the interaction 9 compounds development between lipid oxidation compounds and amine biological 10 constituents (Aubourg, 1998a; Aubourg, 1998b). However, a great diversity of 11 molecules (aldehydes, amines, and so on) could be involved in the fluorescent 12 compounds formation, so that the entire difference in fluorescence development 13 between both species should not be explained as a result of FA presence (Aubourg and 14 Gallardo, 1997; Aubourg, 1998c). 15 16 17 18 CONCLUSIONS 19 20 From the present results, fluorescence detection (Faq value) of interaction 21 compounds formed during the frozen storage of two lean fish species showed to be 22 sensible to quality changes along the storage. Correlation values with the storage time 23 showed to be as good as the FFA determination. 24 Lipid damage measurements have shown differences at –10ºC between both fish 25 species. Haddock showed to be more susceptible to lipid oxidation (PV and TBA-i) and 11 1 hydrolysis (FFA) development than cod. Some complementary research should be 2 carried out in order to assess the interaction between FA and oxidative and hydrolytic 3 enzymes during the frozen storage of FA-forming fish species, and evaluate the relative 4 incidence of this interaction on the general lipid degradation mechanism. Complemntary 5 research also should be carried out to assess the different pathways of FA-lipid 6 oxidation compounds-nucleophilic compounds interaction and the relative effect of each 7 kind of compound in the total fluorescence formation. 8 9 10 11 12 13 ACKNOWLEDGMENTS 14 We thank Mr. Marcos Trigo and Mrs. Montserrat Martínez for technical 15 assistance, Dr. Paul Reece for providing the fish samples and the European Community 16 for financial support of the Research Project FAIR-CT95-1111 (1996-1999). 17 12 REFERENCES 1 2 3 4 5 6 7 8 9 10 Aubourg, S. 1998a. Influence of formaldehyde in the formation of fluorescence related to fish deterioration. Z. Lebensm. Unters. Forsch. 206 29-32. Aubourg, S. 1998b. Effect of pH on the fluorescence formation related to fish deterioration. Z. Lebensm. Unters. Forsch. 207 268-272. Aubourg, S. 1998c. Fluorescence detection in aldehyde containing model systems: Relationship with fish deterioration. Grasas y Aceites 49 419-424. Aubourg, S. and Gallardo, J. 1997. Fluorescence changes in amine model systems related to fish deterioration. Int. J. Food Sci. Technol. 32 153-158. 11 Aubourg, S. and Medina, I. 1997. Quality differences assessment in canned sardine 12 (Sardina pilchardus) by detection of fluorescent compounds. J. Agric. Food 13 Chem. 45 3617-3621. 14 Aubourg, S., Sotelo, C. and Pérez-Martín, R. 1998. Assessment of quality changes in 15 frozen sardine (Sardina pilchardus) by fluorescence detection. J. Am. Oil Chem. 16 Soc. 75 575-580. 17 18 Bligh, E. and Dyer, W. 1959. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 37 911-917. 19 Careche, M. and Tejada, M. 1990. The effect of neutral and oxidised lipids on 20 functionality in hake (Merluccius merluccius L.): A dimethylamine- and 21 formaldehyde-forming species during frozen storage. Food Chem. 36 113-128. 22 23 24 25 Careche, M. and Tejada, M. 1994. Hake natural actomyosin interaction with free fatty acids during frozen storage. J. Sci. Food Agric. 64 501-507. Chapman, R. and McKay, J. 1949. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J. Am. Oil Chem. Soc. 26 360-363 13 1 2 Davies, H. and Reece, P. 1982. Fluorescence of fish muscle: causes of change occurring during frozen storage. J. Sci. Food Agric. 33 1143-1151. 3 de Koning, A. and Mol, T. 1991. Quantitative quality tests for frozen fish: soluble 4 protein and free fatty acid content as quality criteria for hake (Merluccius 5 merluccius) stored at -18C. J. Sci. Food Agric. 54 449-458. 6 7 8 9 10 11 Gardner, H. 1979. Lipid hydroperoxide reactivity with proteins and amino acids: A review. J. Agric. Food Chem. 27 220-229. Han, T. and Liston, J. 1988. Correlation between lipid peroxidation and phospholipid hydrolysis in frozen fish muscle. J. Food Sci. 53 1917-1918. Hardy, R. and McGill, A. 1990. The influence of cold-storage dehydration on the oxidation of white fish. I.I.F.- I.I.R.- Commission E2, Aberdeen (UK), pp. 1-5. 12 Howell, N. 1995. Interaction of proteins with small molecules. In Ingredient 13 interactions -Effects on food quality, ed. Gaoucar, A. Marcel Dekker, New York 14 (USA), pp. 269-289. 15 Howell, N., Shavila, Y., Grootveld, M. and Williams, S. 1996. High-resolution NMR 16 and magnetic resonance imaging (MRI) studies on fresh and frozen cod (Gadus 17 morhua) and haddock (Melanogrammus aeglefinus). J. Sci. Food Agric. 72 49- 18 56. 19 Joly, A., Huidobro, A. and Tejada, M. 1997. Influence of lipids on dimethylamine 20 formation in model systems of hake (Merluccius merluccius) kidney during 21 frozen storage. Z. Lebensm. Unters. Forsch. 205 14-18. 22 Kurade, S. and Baranowski, J. 1987. Prediction of shelf-life of frozen minced fish in 23 terms of oxidative rancidity as measured by TBARS number. J. Food Sci. 52 24 300-302, 311. 14 1 2 Lowry, R. and Tinsley, I. 1976. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 53 470-472. 3 Mackie, I. 1993. The effects of freezing on flesh proteins. Food Rev. Int. 9 575-610. 4 Melton, S. 1983. Methodology for following lipid oxidation in muscle foods. Food 5 6 7 Technol. 37 105-111, 116. Miyashita, K. and Takagi, T. 1986. Study on the oxidative rate and prooxidant activity of free fatty acids. J. Am. Oil Chem. Soc. 63 1380-1384. 8 Orlick, B., Oehlenschläger, J. and Schreiber, W. 1991. Changes in lipids and 9 nitrogenous compounds in cod (Gadus morhua) and saithe (Pollachius virens) 10 11 12 13 14 15 16 17 18 19 20 during frozen storage. Arch. Fisch. Wiss. 41 89-99. Pearson, A., Love, J. and Shorland F. 1977. Warmed-over flavor in meat, poultry and fish. Adv. Food Res. 23 2-61. Pérez-Villarreal, B. and Howgate, P. 1991. Deterioration of European hake (Merluccius merluccius) during frozen storage. J. Sci. Food Agric. 55 455-469. Pigott, G. and Tucker, B. 1987. Science opens new horizons for marine lipids in human nutrition. Food Rev. Int. 3 105-138. Pokorný, J. 1977. Interactions of oxidized lipids with proteins. Riv. Ital. Sostanze Grasse 54 389-393. Quaranta, H. and Pérez, S. 1983. Chemical methods for measuring changes in freeze stored fish: A review. Food Chem. 11 79-85. 21 Rehbein, H. 1988. Relevance of trimethylamine oxide demethylase activity and 22 haemoglobin content to formaldehyde production and texture deterioration in 23 frozen stored minced fish muscle. J. Sci. Food Agric. 43 261-276. 24 Rehbein, H. and Orlick, B. 1990. Comparison of the contribution of formaldehyde and 25 lipid oxidation products to protein denaturation and texture deterioration during 15 1 frozen storage of minced ice-fish fillet (Champsocephalus gunnari and 2 Pseudochaenichthys georgianus). Int. J. Refrig. 13 336-341. 3 4 5 6 Shenouda, S. 1980. Theories of protein denaturation during frozen storage of fish flesh. Adv. Food Res. 26 275-311. Smith, G., Hole, M. and Hanson, S. 1990. Assessment of lipid oxidation in Indonesian salted-dried Marine catfish (Arius thalassinus). J. Sci. Food Agric. 51 193-205. 7 Sotelo, C., Piñeiro, C. and Pérez-Martín, R. 1995. Review. Denaturation of fish proteins 8 during frozen storage: Role of formaldehyde. Z. Lebensm. Unters. Forsch. 200 9 14-23. 10 11 12 13 Statsoft. 1994. Statistica for Macintosh; Statsoft and its licensors, Tulsa, Oklahoma (USA). Vidya Sagar Reddy, G., Srikar, L. and Sudhakara, N. 1992. Deteriorative changes in pink perch mince during frozen storage. Int. J. Food Sci. Technol. 27 271-276. 14 Vyncke, W. 1970. Direct determination of the thiobarbituric acid value in trichloracetic 15 acid extracts of fish as a measure of oxidative rancidity. Fette Seifen Anstrichm. 16 72 1084-1087. 17 16 FIGURE LEGENDS 1 2 3 4 5 6 7 Figure 1: 8 Changes in FFA content (Fig. 1A) and Faq value (Fig. 1B) during cod frozen 9 storage. Mean value and standard deviation are expressed. 10 11 12 13 14 Figure 2: 15 Changes in FFA content (Fig. 2A) and Faq value (Fig. 2B) during haddock 16 frozen storage. Mean value and standard deviation are expressed. 17 18 19 17 TABLE 1: Lipid damage measurement* during cod frozen (-30C) storage** ST FFA PV TBA-i For Faq 0 71.4 ab 2.0 a 0.11 a 0.75 ab 0.82 a 1 58.9 a 2.6 a 0.12 a 0.83 b 1.03 ab 3 94.2 ab 2.8 a 0.38 b 0.64 ab 0.98 a 5 104.9 bc 3.7 ab 0.39 b 0.67 ab 1.17 ab 7 195.0 d 3.4 ab 0.19 a 1.43 c 1.48 ab 9 185.6 d 5.2 b 0.41 b 0.60 ab 5.87 c 12 143.1 c 7.9 c 0.49 b 0.50 a 4.75 bc * Means of three independent determinations. Values in the same column followed by different letters are significantly different (p<0.05). ** Abbreviations and units: ST (storage time; months), FFA (free fatty acids; g FFA kg-1 lipids), PV (peroxide value; meq oxygen kg-1 lipids), TBA-i (thiobarbituric acid index; mg malondialdehyde kg-1 sample), For (fluorescence shift in organic phase) and Faq (fluorescence shift in aqueous phase) (fluorescence determinations calculated as expressed in the Materials and Methods section). TABLE 2: Lipid damage measurement* during cod frozen (-10C) storage** ST FFA PV TBA-i For Faq 0 71.4 a 2.0 a 0.11 ab 0.75 a 0.82 a 1 268.0 b 3.3 a 0.10 ab 0.69 a 1.07 a 3 394.9 c 6.5 b 0.43 d 0.66 a 1.04 a 5 510.0 de 6.7 b 0.61 e 1.07 ab 1.44 ab 7 451.3 cd 6.2 b 0.07 a 1.26 b 6.82 b 9 556.8 de 7.8 b 0.28 cd 1.00 ab 15.78 c 12 488.4 d 1.7 a 0.26 bc 1.05 ab 17.87 c * Means of three independent determinations. Values in the same column followed by different letters are significantly different (p<0.05). ** Abbreviations and units as indicated in Table 1. 19 TABLE 3: Lipid damage measurement* during haddock frozen (-30C) storage** ST FFA PV TBA-i For Faq 0 89.6 a 1.8 a 0.26 ab 0.77 a 0.62 a 1 93.8 a 3.2 a 0.17 a 0.69 a 0.80 a 3 151.3 bc 3.0 a 0.35 b 0.79 a 0.78 a 5 122.1 ab 5.2 b 0.45 b 0.80 a 0.78 a 7 189.4 cd 5.8 bc 0.42 b 2.10 b 4.88 b 9 210.4 d 7.6 cd 0.66 c 0.78 a 3.34 ab 12 211.1 d 8.5 d 0.42 b 0.67 a 5.51 b * Means of three independent determinations. Values in the same column followed by different letters are significantly different (p<0.05). ** Abbreviations and units as indicated in Table 1. 20 TABLE 4: Lipid damage measurement* during haddock frozen (-10C) storage** ST FFA PV TBA-i For Faq 0 89.6 a 1.8 a 0.26 a 0.77 a 0.62 a 1 296.0 b 7.2 ab 0.18 a 0.79 a 0.83 a 3 475.9 c 8.5 b 0.60 ab 1.19 a 0.97 a 5 449.7 c 9.6 b 0.81 b 1.13 a 0.78 a 7 464.7 c 8.6 b 0.30 a 1.75 b 1.13 a 9 555.8 d 30.5 c 0.61 ab 1.85 b 6.66 b 12 542.4 d 0.8 a 0.31 a 1.02 a 10.94 b * Means of three independent determinations. Values in the same column followed by different letters are significantly different (p<0.05). ** Abbreviations and units as indicated in Table 1. 21 TABLE 5: Linear correlation matrix* for different parameters (storage time and lipid damage indices) measured during cod frozen (-30ºC) storage** ST PV TBA-i FFA For Faq 0.83* 0.67* 0.71* (0.75*) -0.05 0.66* (0.74*) 0.69* 0.33 -0.32 0.54* 0.22 -0.50* 0.46 0.37 0.51* PV TBA-i FFA For -0.24 * Significant (p<0.05) values. ** Abbreviations as specified in Table 1. Results in brackets correspond to nonlinear fittings (logarithmic for FFA; exponential for Faq). 22 TABLE 6: Linear correlation matrix* for different parameters (storage time and lipid damage indices) measured during cod frozen (-10ºC) storage** ST PV TBA-i FFA For Faq 0.17 0.17 0.79* (0.91*) 0.46* 0.88* (0.91*) 0.39 0.61* 0.24 0.02 0.44 0.09 -0.01 0.38 0.54* PV TBA-i FFA For 0.43 * Significant (p<0.05) values. ** Abbreviations as specified in Table 1. Results in brackets correspond to nonlinear fittings (logarithmic for FFA; exponential for Faq). 23 TABLE 7: Linear correlation matrix* for different parameters (storage time and lipid damage indices) measured during haddock frozen (-30ºC) storage** ST PV TBA-i FFA For Faq 0.93* 0.66* 0.85* (0.84*) 0.14 0.70* (0.78*) 0.55* 0.67* 0.20 0.61* 0.77* 0.08 0.33 0.23 0.68* PV TBA-i FFA For 0.46* * Significant (p<0.05) values. ** Abbreviations as specified in Table 1. Results in brackets correspond to nonlinear fittings (logarithmic for FFA; exponential for Faq). 24 TABLE 8: Linear correlation matrix* for different parameters (storage time and lipid damage indices) measured during haddock frozen (-10ºC) storage** ST PV TBA-i FFA For Faq 0.26 0.16 0.84* (0.93*) 0.51* 0.74* (0.81*) 0.40 0.43 0.66* 0.13 0.36 0.30 -0.07 0.58* 0.53* PV TBA-i FFA For 0.10 * Significant (p<0.05) values. ** Abbreviations as specified in Table 1. Results in brackets correspond to nonlinear fittings (logarithmic for FFA; exponential for Faq). 25 TABLE 5: Linear correlation values for the storage time and the different lipid indices** during the frozen storage (-30C and -10C) of both species (cod and haddock)*** Measurement Fish species (frozen storage temperature) Cod (-30C) Cod (-10C) Haddock (-30C) Haddock (-10C) FFA 0.71* (0.75*) 0.79* (0.91*) 0.85* (0.84*) 0.84* (0.93*) PV 0.83* 0.17 0.93* 0.26 CD 0.65* 0.59* 0.72* 0.55* TBA-i 0.67* 0.17 0.66* 0.16 For -0.05 0.46* 0.14 0.51* Faq 0.66* (0.74*) 0.88* (0.91*) 0.70* (0.78*) 0.74* (0.81*) * Significant (p<0.05) values. ** Abbreviations as specified in Table 1. *** Results in brackets correspond to nonlinear fittings (logarithmic for FFA and ST; exponential for Faq and ST). 26