Elements and Compounds
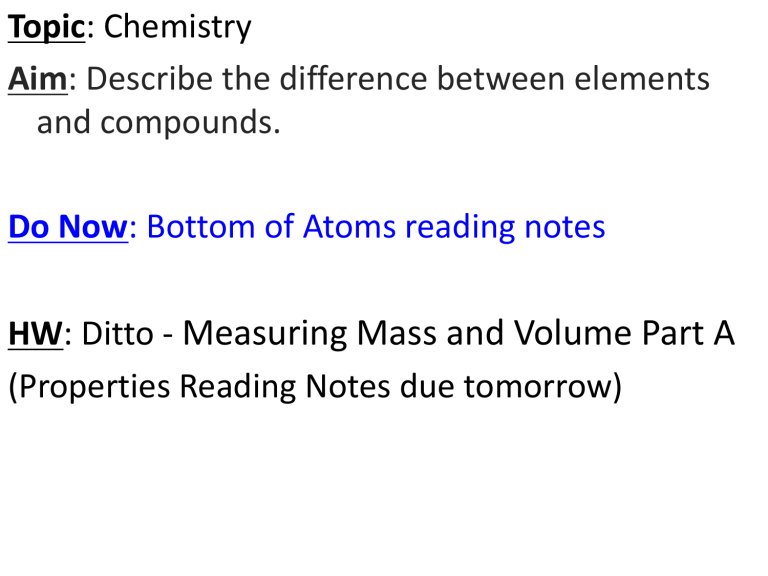
Topic: Chemistry
Aim: Describe the difference between elements and compounds.
Do Now: Bottom of Atoms reading notes
HW: Ditto Measuring Mass and Volume Part A
(Properties Reading Notes due tomorrow)
Let’s review…
1. Identify the building blocks of matter.
2. Identify the subatomic particles that make up an atom.
3. Describe the location of each subatomic particle.
4. Describe the charge of each subatomic particle.
5. What is an atom’s atomic number indicate?
Oxygen atom
+
-
Oxygen atom
-
-
-
Electron
+
-
+
+
+
Nucleus
-
+
+
+
+
-
Neutron
-
Proton
-
Elements
• Made up of only ONE type of atom
• CANNOT be broken down
26
79
29
47
6
28
17
3 atoms
2 elements
3 atoms
2 elements
Compounds 1. How many atoms are
4 atoms
2 elements found in each compound?
2. How many elements are found in each compound?
3. How are the atoms connected to
5 atoms each other?
2 elements
Compounds
• Made up of 2 or more different elements CHEMICALLY combined
• CAN BE BROKEN DOWN
Compounds have properties DIFFERENT from the properties of the elements that it is made up of.
• Compounds have properties DIFFERENT from the elements it is made up of
Na Cl NaCl
Molecule
•
Formed when 2 or more ATOMS join together chemically
•
All compounds are molecules
Water is a compound because it is made up of the elements H and O.
This is one molecule of water because H and O are bonded together.
When atoms of the same element join together we get a molecule of that element.
Oxygen (O
2
)
Hydrogen (H
2
)
Compounds will always exist as molecules, not separate atoms.
3 water (H
2
O) molecules
2 carbon dioxide (CO
2
) molecules
Ex: A glass of water is made up of millions of water molecules that are exactly the same
1 molecule of
Sodium chloride
Thousands of molecules of
Sodium chloride
Let ’ s summarize:
1. Describe an element.
2. Describe a compound.
3. Describe the difference between an element and a compound.
4. Are all compounds molecules? Support your answer.
5. Identify a molecule that is not a compound.
Review:
An object has a mass of 10g and a volume of 2cm 3 . Identify the density of the object.
D = M/V
10g/2cm 3
= 5g/cm 3
Identify the instrument used to measure mass.
1.Thermometer
2.Graduated cylinder
3.Triple beam balance
4.Metric ruler
The SI units used when measuring mass are
1.milliliters
2.centimeters cubed
3.meters
4.grams
Identify the instrument used to measure temperature.
1.Thermometer
2.Graduated cylinder
3.Triple beam balance
4.Metric ruler
Identify the SI units used to measure temperature.
1.meter
2.degrees Celsius
3.grams
4.Degrees Fahrenheit
Identify the SI units used to measure the density of a liquid.
1.mL
2.g/cm 3
3.cm
3
4.g/mL







