jovani_trends_parasitol_02.doc
advertisement

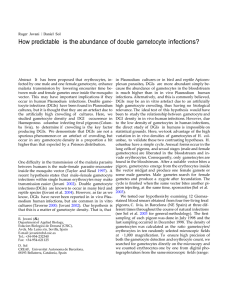
Malaria transmission, sex ratio and erythrocytes with two gametocytes Roger Jovani Transmission of haemospororin parasites (phylum Apicomplexa) needs the fertilization of at least one female by one male gamete within the bloodmeal of a suitable vector. Male and female gamete precursors (gametocytes) in Plasmodium and Haemoproteus parasites are normally alone inside the erythrocytes of the vertebrate host, but they also occur in male–female pairs in single erythrocytes. These paired gametocytes could enhance transmission success by facilitating the encounter between the female and male gametes when inside the midgut of the vector. Further study of these particular infections could provide new insights into the biology of and control strategies for haemospororin parasites. Roger Jovani Dept Applied Biology, Estación Biológica de Doñana, Consejo Superior de Investigaciones Científicas, Avda. Ma Luisa s/n, 41013 Sevilla, Spain. e-mail: jovani@ebd.csic.es With the bite of an infected blood-feeding dipteran vector, sporozoites of haemospororin parasites (phylum Apicomplexa; Plasmodium, Haemoproteus and Leucocytozoon) travel to and infect the tissue cells of the vertebrate host, and mature to an asexual schizont stage. Merozoites then emerge from the schizont and invade erythrocytes, producing (sexual) gametocytes or continuing schizogony until new merozoites are determined to be gametocytes. For Plasmodium falciparum, all merozoites from the same schizont are either all sexual or all asexual [1], and gametocytes are either all male or all female [2]. Transmission begins when a suitable vector bites an infected host. Mature male and female gametocytes emerge from red blood cells of the bloodmeal in the midgut of the vector, producing up to eight mobile male gametes (exflagellation) or differentiating into a single, static, female gamete (rounding up). Fertilization occurs when a male gamete finds and fuses with a female gamete. After fertilization, the zygote differentiates into an invasive ookinete that will penetrate the midgut epithelium of the vector. There, the ookinete becomes an oocyst, which will finally produce thousands of sporozoites, infecting a new host on a subsequent bite by the vector. Although haemospororin gametocytes are normally alone inside erythrocytes, double-gametocyte infections (DGIs) occur in Plasmodium and Haemoproteus parasites [3,4]. However, the importance of this phenomenon for the biology and control of malaria has not been considered. The DGI hypothesis states that the encounter time between male and female gametes of a male–female DGI in the midgut of the dipteran vector is less than the time needed for those gametes emerging from singly infected erythrocytes; therefore, in scenarios of constrained transmission by single gametocytes, male–female DGIs would have a key role. In Plasmodium spp., the low density of gametocytes [5] and the low proportion of gametocytes reaching the ookinete stage [6] indicate that fertilization is a major bottleneck on transmission. Thus, a male–female DGI would enhance transmission success. Current knowledge about DGIs The ‘DGI hypothesis’ has two prerequisites: (1) male–female DGIs must occur in natural infections; and (2) male–female DGIs must be viable. DGIs have never been reported for infections of Leucocytozoon spp., although studies actively searching for DGIs in these species are needed. In natural infections of Haemoproteus spp., mature male–female DGIs have been reported for birds and tortoises [4,7]. DGIs are also found in cultures of P. falciparum and other Plasmodium spp. [3], but have not been reported in natural Plasmodium infections. However, the static environment into which merozoites are normally released in vitro could not explain the lack of information about DGIs in natural infections of some Plasmodium spp. This is because, in in vivo infections of P. falciparum and other Plasmodium spp., microvascular obstruction resulting from cytoadherence (binding of infected erythrocytes on the endothelium of capillaries and venules) and rosetting (adherence of late-stage asexual infected erythrocytes to uninfected red blood cells; Fig. 1) also creates an environment with little or no blood flow when merozoites emerge from infected erythrocytes [8]. Why, therefore, are DGIs not reported in natural Plasmodium infections? First, gametocytaemia is much higher in natural infections of Haemoproteus and in Plasmodium cultures than in natural infections of Plasmodium spp. Second, DGIs are rarely considered in publications. For instance, in Haemoproteus columbae, DGIs have been reported in some studies [7,9] but, when it does occur, it is treated as an anecdotal phenomenon [7] or is not discussed in the text even when it does appear in the figures [9]. Alternatively, DGIs might really not occur in natural infections of Plasmodium spp. However, multiple asexual infections inside single erythrocytes have been seen in many in vivo infections of Plasmodium spp. (e.g. P. falciparum and Plasmodium vivax [10]), and they are also produced by two merozoites entering a given erythrocyte. However, the important question is not whether DGIs could occur in natural infections, but whether male– female DGIs could occur. Two non-exclusive models could explain how male–female DGIs could be produced in P. falciparum (Fig. 1). Even less is known about the viability of DGIs in vivo than about their occurrence. However, DGIs have been found to reach maturity in cultures of P. falciparum and other Plasmodium spp., and in natural Haemoproteus infections [3,7]. Moreover, some mature (stage V) (a) (b) TRENDS in Parasitology Fig. 1. Two hypothetical models of how male–female double-gametocyte infections (DGIs) could be produced by Plasmodium falciparum in human capillaries. Adhered and non-adhered erythrocytes to the schizont are assumed to be susceptible to merozoite invasion, following the model proposed in Ref. [25]. Schizonts coming from singly (a) or doubly (b) asexually infected erythrocytes are indicated in grey. Because of the lack of information on ‘double schizonts’ [24], they have been assumed to produce a similar number of merozoites as ‘single schizonts’. Schizonts adhere to the endothelium of the capillary and show rosetting with uninfected erythrocytes (red). Merozoites committed to be male and female gametocytes are represented by black and white dots, respectively, so male–female DGIs result from those erythrocytes (red) with a black and a white dot. female–female DGIs have been seen in P. falciparum cultures and, on one occasion, one female gametocyte from a female–female DGI has been seen to round up (L. Baton and L. Ranford-Cartwright, pers. commun.). It is particularly interesting that the technique used to produce these results (Giemsa-stained smears from a P. falciparum culture) was not designed specifically to find DGIs or exflagellation/rounding up processes. Thus, although they are anecdotal, these data suggest that male–female DGIs could also be viable. meeting in the bloodmeal [17] and (2) host antibodies in the bloodmeal agglutinate male gametes, reducing male-gamete motility [18]. In addition, each male gametocyte produces few male gametes (around four in P. falciparum), imposing a lower sex-ratio limit because f the need to produce sufficient male gametes to fertilize the female gametes [19]. However, there are some groups of blood-dwelling apicomplexan parasites to which this theoretical framework could not be applied because of ‘syzygy’ – male and female gametocytes pairing before gametogenesis. Syzygy occurs in blood-dwelling adeleorins and piroplasms, and the available data supports the prediction that syzygy favours a sex ratio of 0.5 [20]. Syzygy could be seen as an extreme point of DGI, at which all gametocytes are in male–female DGIs. Thus, the sex ratio is predicted to become less female biased as the proportion of fertilizations coming from male–female DGIs increases, maximizing the male–female gametocyte ratio rather than the male–female gamete ratio. If we do not take into account the importance of fertility insurance [17], the optimal sex ratio (r*) could be defined by Eqn 1, r* = [(1 − F) ÷ 2]s + 0.5d [Eqn 1] and the lower sex ratio limit could be given by Eqn 2, Low gametocyte densities When they are present, P. falciparum gametocytes are found in very low densities (146–485 gametocytes µl−1 [5]), and a very low proportion reaches the ookinete stage (40– 1223-times reduction in in vitro cultures, i.e. out of 40 gametocytes, only one reaches the ookinete stage). However, this does not seem to be an insuperable problem for the transmission of P. falciparum, because people with a gametocytaemia under the detection level of three gametocytes µl−1 have been found to be infective to mosquitoes [11–14], even at a similar proportion to positive gametocyte carriers [12]. How are these very low gametocyte levels infective? The number of gametocytes in different bloodmeals from the same person shows an aggregate distribution, some bloodmeals having a fivefold higher density than the mean [15]. However, the DGI hypothesis states that only one male–female DGI in a bloodmeal is necessary for transmission, being much more efficient than one male and one female gametocyte in singly infected erythrocytes. Hence, these two complementary processes could act simultaneously, enhancing transmission success in situations of low gametocytaemias. Could DGI explain apicomplexan sex ratios? Current theory about the sex ratio (the proportion of male gametocytes) in apicomplexan parasites states that this is shaped by two opposing forces [16]. The first is inbreeding, which produces female-biased sex ratios. The second is ‘fertility insurance’, which produces more equal sex ratios under transmission-compromised scenarios, because (1) the low number of gametocytes leads to a low probability of a male and a female gamete r* = [(1 ÷ (1 + c)]s + 0.5d [Eqn 2] where s is the proportion of fertilizations between gametes that do not come from male–female DGIs (most of them from singly infected erythrocytes), d is the proportion of fertilizations from male–female DGIs (i.e. d + s = 1), F is the inbreeding rate and c is the mean number of gametes produced by each male gametocyte (Fig. 2). Therefore, the DGI hypothesis predicts that haemospororin parasites will benefit from adjusting their sex ratio depending on the value of d. Accordingly, in Leucocytozoon parasites (which probably have d = 0), the inbreeding probability has a significant negative correlation with sex ratio [21]. If the DGI hypothesis is true, Plasmodium and Haemoproteus parasites would be expected to have d values that are much higher than the proportion of male–female DGIs in the bloodstream, owing to a higher fertilization efficiency of male–female DGIs compared with single-gametocyte infections. This could be especially relevant at low gametocyte densities (because this lowers the probability of encounters between male and female gametes coming from single gametocytes, but does not affect fertilization between gametes from a male–female DGI) and when male gamete motility is impeded by the action of antibodies [18] (because only male gametes from singly infected erythrocytes, but not from male–female DGIs, need to travel to encounter the female gamete and therefore could be affected by host agglutination antibodies). Optimal sex ratio (r*) Fig. 2. Optimal sex ratio (r*) plotted against inbreeding rate (F ) for different proportions of fertilizations coming from double-gametocyte infections (d ). This assumes that a mean of four gametes emerge from each male gametocyte. 0.5 d=1 d = 0.8 0.4 d = 0.6 d = 0.4 0.3 d = 0.2 d=0 0.2 0.1 0 0 0.2 0.4 0.6 0.8 1 Inbreeding rate (F) TRENDS in Parasitology Acknowledgements I thank Lisa RanfordCartwright for a fruitful discussion. Jordi Torres first alerted me to the existence of DGIs. I thank Daniel Sol, Jordi Torres, José Luis Tella, David Serrano, Dave Shutler and Anna Perera for continuous talks during the gestation of this idea. Andrea Kraljevic, José María Fedriani and Begoña Martinez corrected the English. Andrew Read and two anonymous referees improved the article. The sex ratio of Haemoproteus spp. has been found to be between 0.3 and 0.42, which did not show a statistical relationship with the probability of inbreeding [22]. In five populations with very low prevalence (<20%), and so predicted to have a very high inbreeding rate [21], the sex ratios were 0.35, 0.36, 0.40, 0.41 and 0.42, independent of inbreeding rate [22]. Thus, supposing a relatively high inbreeding rate of F > 0.5, Eqns 1 and 2 predict that ~40% of the fertilizations could come from male– female DGIs (Fig. 2) if Haemoproteus lineages could adjust their sex ratio. Therefore, the DGI hypothesis predicts for the first time a sex ratio close to 0.4, and independent of inbreeding rate, in situations with moderate to high proportions of fertilization coming from DGIs (d = ~0.4). References 1 Bruce, M.C. et al. (1990) Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 100, 191–200 2 Smith, T.G. et al. (2000) Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology 121, 127–133 3 Taverne, J. (2000) ParaSite: how many gametocytes can fit into one red blood cell? Parasitol. Today 16, 48–49 4 Lainson, R. and Naiff, R.D. (1998) Haemoproteus (Apicomplexa: Haemoproteidae) of tortoises and turtles. Proc. R. Soc. London B Biol. Sci. 265, 941–949 5 Taylor, L.H. and Read, A.F. (1997) Why so few transmission stages? Reproductive restraint by malaria parasites. Parasitol. Today 13, 135–140 6 Ghosh, A. et al. (2000) The journey of the malaria parasite in the mosquito: hopes for the new century. Parasitol. Today 16, 196–201 7 Bennett, G.F. and Peirce, M.A. (1990) The haemoproteid parasites of the pigeons and doves (family Columbidae). J. Nat. Hist. 24, 311–325 8 Kaul, D.K. et al. (1991) Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood 78, 812–819 9 Mushi, E.Z. et al. (1999) Haemoproteus columbae in domestic pigeons in Sebele, Gaborne, Botswana. Onderstepoort J. Vet. Res. 66, 29–32 Plasmodium spp. vary greatly in their sex ratio, both between species and during the course of infections, with a general tendency to increase during late infections (characterized by low gametocytaemias) [23] and when the motility of male gametes is decreased by host antibodies [18]. This suggests that, whereas DGIs could be producing an important and constant proportion of fertilizations in Haemoproteus spp., it could be playing a key role in the transmission success of Plasmodium spp., particularly under scenarios of highly constrained transmission. Future directions The occurrence and viability of DGIs are not well established. We therefore need to measure the proportion of male–female DGIs and the sex ratio in hosts with different gametocytaemias (particularly low ones) of different haemospororin spp. Moreover, the viability of DGIs needs to be studied and compared with the viability of single-gametocyte infections. If DGIs are playing a role in malaria transmission, the design of vaccines against malaria parasites must take this into account. For instance, antibodies against merozoite surface antigens of P. falciparum enhance the production of multiply infected erythrocytes [24], and so antibodies might also be promoting DGIs and possibly enhancing malaria transmission. If the DGI hypothesis is true, it will have profound consequences on our understanding of the biology of haemospororin parasites and improve control strategies, such as the design of vaccines. Therefore, a test of the DGI hypothesis merits international consideration. 10 Simpson, J.A. et al. (1999) Red cell selectivity in malaria: a study of multiple-infected erythrocytes. Trans. R. Soc. Trop. Med. Hyg. 93, 165–168 11 Gamage-Mendis, A.C. et al. (1991) Infectious reservoir of Plasmodium vivax and Plasmodium falciparum malaria in an endemic region of Sri Lanka. Am. J. Trop. Med. Hyg. 45, 479–487 12 Boudin, C. et al. (1993) High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am. J. Trop. Med. Hyg. 48, 700–706 13 Robert, V. et al. (1995) Detection of falciparum malarial forms in naturally infected anophelines in Cameroon using a fluorescent anti-25-kD monoclonal antibody. Am. J. Trop. Med. Hyg. 52, 366–369 14 Bonnet, S. et al. (2000) Comparison of artificial membrane feeding with direct skin feeding to estimate infectiousness of Plasmodium falciparum gametocyte carriers to mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 94, 103–106 15 Pichon, G. et al. (2000) High heterogeneity in the number of Plasmodium falciparum gametocytes in the bloodmeal of mosquitoes fed on the same host. Parasitology 121, 115–120 16 West, S.A. et al. (2001) Evolution of gametocyte sex ratios in malaria and related apicomplexan (protozoan) parasites. Trends Parasitol. 17, 525–531 17 West, S.A. et al. (2002) Fertility insurance and the sex ratios of malaria and related haemospororin blood parasites. J. Parasitol. 88, 258–263 18 Paul, R.E.L. et al. (2000) Sex determination in malaria parasites. Science 287, 128–131 19 Read, A.F. et al. (1992) Gametocyte sex ratios as indirect measures of outcrossing rates in malaria. Parasitology 104, 387–395 20 West, S.A. et al. (2000) Sex allocation and population structure in apicomplexan (protozoa) parasites. Proc. R. Soc. London B Biol. Sci. 267, 257–263 21 Read, A.F. et al. (1995) Sex allocation and population structure in malaria and related parasitic protozoa. Proc. R. Soc. London B Biol. Sci. 260, 359–363 22 Shutler, D. et al. (1995) Sex proportions of Haemoproteus blood parasites and local mate competition. Proc. Natl. Acad. Sci. U. S. A. 92, 6748–6752 23 Paul, R.E.L. et al. (2002) Plasmodium sex determination and transmission to mosquitoes. Trends Parasitol. 18, 32–38 24 Ramasamy, R. et al. (2001) Antibodies and Plasmodium falciparum merozoites. Trends Parasitol. 17, 194–197 25 Clough, B. et al. (1998) The role of rosetting in the multiplication of Plasmodium falciparum: rosette formation neither enhances nor targets parasite invasion into uninfected red cells. Br. J. Haematol. 100, 99–104