Nuclear 2
advertisement

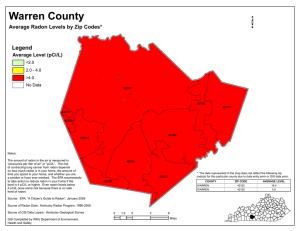
Radiochemistry Concepts and Applications in Environmental Chemistry Isotopes Too heavy, too light, rarely just right. Table of Isotopes 1998 Version, 3000+ isotopes Properties of , , and Properties of , , and ALPHA Symbol , 42He 2+ Charge +2 Mass (kg) 6.642x10-27 Velocity 0.05c (c=2.998x108m/s) Relative Ionization 1x104 Potential Relative Penetrating 1 Potential BETA X-, & GAMMA , 0-1, 0-1e and -1 0 9.116x10-31 0 up to 0.995c c 1x102 1 1x102 1x104 Penetration Potential Ionization of Gas Ionization of Liquids Scintillation Detection Solid-State Semiconductor Detectors HPGe Detector Structure CANBERRA Analytical Nuclear Instruments Ionization of Solids Spectroscopy with HPGe “Ultra-Low” Background Considerations Cosmogenic - from the cosmos Auroras Naturally Occurring Radioactive Materials (“NORM”) Cosmogenic: 3 H 7,10 Be 35,38 S 14 C 18 F 36,38,39 Cl 22,24 Na 26Al 37,39 Ar 53Mn 31,32 59 Si Ni 80 32,33 P Kr Primordial: 40 K 50V 87Rb 113Cd 115In 148 Sm 152Gd 174Hf 176Lu 232 Th+daughters 235 123 Te 187 Re 138 La 144Nd 147Sm 190,192 Pt 209Bi U+daughters 237Np+daughters 238 U+daughters Localized “Hot Spots” Location Annual Dose(mSv/y) United Arab Emirates Radon Springs, France Kerala Region of India Guangdong Province, China Morro De Faro, Brazil Guarapari, Brazil Ramasari, Iran USNRC Occupational Dose Limit USNRC Limit for Members of Public 14 16 30 33 70 - 140 175 480 50 20 Oklo Quarry, Gabon Fossil Fission Reactor Oklo Quarry, Gabon In 1972 ore deposits were found to contain significantly different isotopic compositions of certain elements than from the mean found in nature. 142Nd normally 27% in nature, at Oklo it was <6%. 99Ru normally ~28% in nature, at Oklo it was 13%. 235U normally 0.72%, at Oklo it was 0.48%. From 87Rb/87Sr dating, Oklo deposits are 1.7E9 years old, at that time U enrichment was ~3% 235U! Fossil Fission Reactor Operated on and off at ~100kW output for 1E6 years. Once the natural reactors burned themselves out, the highly radioactive waste they generated was held in place deep under Oklo by the granite, sandstone, and clays surrounding the reactors’ areas Significance today with Yucca Mountain proposed national repository. Radioactive Material is Everywhere Cosmic Radiation Indoor Air & Structural Materials Rock & Soil Radiation Water & Aquatic Food Ingestion Food, milk digestion Crop digestion Inhalation Skin Absorption “Reference Man” with a 70Kg. Body Mass Nuclide Daily Intake of Nuclides Uranium Total Mass of Nuclide Found in the Body 90 g Thorium 30 g 3 g Radium 31 g 2.3 g Carbon-14 95 g 2 g Potassium-40 17 mg 0.39 mg Tritium 0.06 g 3 g Polonium 0.02 g 0.6 - 5 g* Assumes a smoker. * 1.9 g Sources of Radiation Exposure -Update Release of NRCP No. 160 (03/03/09) Exposure Category Effective Dose per Individual in the US Population (mSv) (1) 2006 (2) Early 1980s Ratio (1)/(2) Ubiquitous Background 3.11 3.00 1.04 Medical 3.00 0.53 5.67 Consumer 0.13 0.13 --- Industrial, educational, research 0.003 0.001 --- Occupational 0.005 0.009 --- Total 6.25 3.67 1.70 Energy Liberated can be Absorbed Absorption in body tissue may result in physiological injury Absorption is the principle by which detection is based. The degree of absorption or type of interaction is a primary factor in determining shielding requirements. Health Effects of Ionizing Radiation Direct Effect on Cells: damage to DNA from ionization. If the cell is exposed to radiation, the probability of the radiation interacting with the DNA is very small since these critical components take up less than 0.5% of the cell volume. USDOE: Human Genome Project Health Effects of Ionizing Radiation Radiolysis of water produces the following types of sequences: H2O + ray HOH+ + e- H2O + e- HOHHOH+ H+ + OH* HOH- H* + -OH OH* + OH* H2O2 (hydrogen peroxide) Net Effect: Free radical formation Indirect Effect on Cells: decomposition of water. If the cell is exposed to radiation, the probability of interaction with cellular water is greater since water is 99.5% of the cell volume. Risk Models ALARA The basic tenants of ALARA are the use of time, distance, and shielding to minimize radiation exposures. Radon Target Organ - Lungs Radon (Rn) and Daughter Products (RDPs) inhaled or ingested. Most of the Rn from respiration is exhaled. Ingested Rn out-gasses through the lungs. RDPs remain stuck to lung tissue. Po218 & Po214 emit alpha particles within the 1st hour. Alpha Particle in the Lung Alpha particles strike lung cells causing possible physical and/or chemical damage. 3 fates for cell • Damaged & Repaired • Damaged, not Repaired • Killed 48 hour time lapsed microscopic photograph of alpha tracks emitted from a radioactive particle of Pu-238 lodged in the lung tissue of an ape. www.ccnr.org/alpha_in_lung.html Iowa Radon Lung Cancer Study Found excess risk of 50% for exposures that are equivalent to 15-years spent at an average radon exposure of 4 pCi/L. Overall, the risk estimates obtained in the study suggest that cumulative Radon exposure in the residential environment is significantly associated with lung cancer risk. Field, R.W., Lynch, C.F., Brus, C.P., Woolson, R.F., Fisher, E.F., Platz, C.E., Robinson, R.A., Steck, D.J., Neuberger, J.S. Residential Radon Gas Exposure and Lung Cancer: The Iowa Radon Lung Cancer Study, American Journal of Epidemiology, 151(11);1091-1102, 2000. Radon Geology Source Rn is constantly being generated by the uranium in rocks, soil, water, and construction materials derived from rocks and soil. Uranium is found in small concentrations throughout the earth’s crust. On average, 1 acre down to a 5 ft. depth would contain 50 lbs. of Uranium. www.atral.com/U238.html Radon Potential Uranium Potential Actual Radon Zones Radon Migration Typical Indoor Air Rn range - 1 to 1000 pCi/L. Typical Soil Air Rn range - 200 to 100,000 pCi/L. Dissolved Rn in Groundwater range - 100 to 3,000,000 pCi/L. High Variability in Radon Concentration Nuclear Generating Plants Every form of energy generation has advantages and disadvantages. Coal Nuclear Advantages Fuel is inexpensive Easy to recover (in U.S. and China) Advantages Fuel is inexpensive Energy generation is the most concentrated source Waste is more compact than any source Extensive scientific basis for the cycle Easy to transport as new fuel No greenhouse or acid rain effects Disadvantages Requires larger capital cost because of emergency, containment, radioactive waste and storage systems Requires resolution of the long-term high level waste storage issue in most countries Potential nuclear proliferation issue Disadvantages Requires expensive air pollution controls (e.g. mercury, sulfur dioxide) Significant contributor to acid rain and global warming Requires extensive transportation system “Clean Coal” does not yet exist New NGP designs - ABWR New NGP designs - PBMR Westchester County Dept. of Labs & Research is a FRMAC partner Laboratory and Personnel Assets are registered in the event of an emergency