Request to Use Hospital Equipments Resources for Research Projects
advertisement
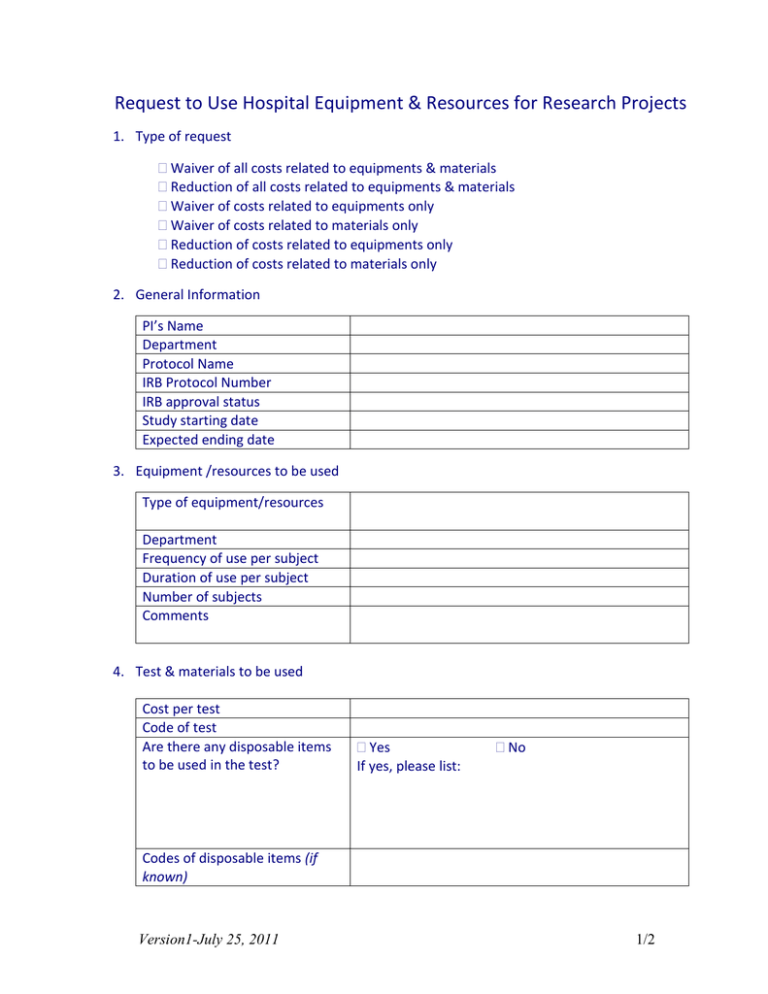
Request to Use Hospital Equipment & Resources for Research Projects 1. Type of request Waiver of all costs related to equipments & materials Reduction of all costs related to equipments & materials Waiver of costs related to equipments only Waiver of costs related to materials only Reduction of costs related to equipments only Reduction of costs related to materials only 2. General Information PI’s Name Department Protocol Name IRB Protocol Number IRB approval status Study starting date Expected ending date 3. Equipment /resources to be used Type of equipment/resources Department Frequency of use per subject Duration of use per subject Number of subjects Comments 4. Test & materials to be used Cost per test Code of test Are there any disposable items to be used in the test? Yes If yes, please list: No Codes of disposable items (if known) Version1-July 25, 2011 1/2 5. Human Resources Is there any clinical staff involvement? Number of involved clinical staff How you intend to Manage staff time between clinical care & research tasks? Please Comment Is there any compensation for staff? Amount of compensation How are you planning to cover this compensation? Yes No Yes No Partial None 6. Funding resources Do you have any type of funding resource? Name of funding resource Amount of available funding 7. Endorsement of the service Department Chair Yes No Name & signature of the Department Chair: -------------------------------------------8. Hospital administration Decision Approval for: Waiver of all costs related to equipments & materials Reduction of all costs related to equipments & materials % reduction --------or New rate---------- Waiver of costs related to equipments only Waiver of costs related to materials only Reduction of costs related to equipments only % reduction --------or New rate---------- Reduction of costs related to materials only % reduction --------or New rate---------- Disapproval Comments: ---------------------------------------------------Signature: ------------------------------------------------------ Version1-July 25, 2011 2/2