Example Poster
advertisement

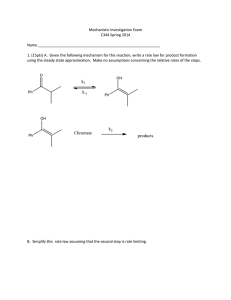
MECHANISTIC STUDIES OF SULFATE HYDROLYSIS 1 Burlingham, 2 Pratt, 3 Davidson, 4 Jr., 2 Fong, 4 Widlanski Benjamin T. Lisa M. Ernest R. Vernon J. Shiner, Jon Theodore S. 1Mount Union College, Department of Chemistry, 1972 Clark Ave., Alliance, OH 44601; 2Indiana University, Bloomington, 3 Department of Geology, Bloomington, IN, 47405; University of Washington, Department of Chemistry, Seattle, WA 981954 1700; Indiana University, Bloomington, Department of Chemistry, Bloomington, IN, 47405. Abstract Materials and Methods A stable isotope mass spectrometry method for the determination of S-34 kinetic isotope effects in sulfate monoester hydrolysis is described. Hydrolysis of aryl sulfates under acidic conditions give large, normal S-34 kinetic isotope effect (KIE) data. These data, along with inverse solvent isotope effects, are inconsistent with the currently proposed concerted mechanism involving simultaneous cleavage and proton transfer in the transition state. This method for the acquisition of S-34 kinetic isotope effects may also prove useful for studying the mechanism of other sulfuryl group transfers, including sulfatase and sulfotransferase catalyzed reactions. Introduction • Sulfate ester hydrolysis important in physiological processes such as desulfation of estrone sulfate • Mechanism of sulfate hydrolysis still not completely understood • O-18 and N-15 KIE previously reported and a mechanism proposed1 Substrate Synthesis of pure sulfate monoesters SO3. pyridinium HO NH R pyridine O O S O O R Silica gel chromatography 2% TEA/acetonitrile K O O S O O AG50W-X8 Dowex. potassium form R O O S O O (Et)3NH R R = -NO2, or -COCH3, Partial acid hydrolysis of sulfate monoesters O O S O O NO2 O O S O O O 1 N HCl - HSO4 1 N HCl - HSO4 CH3 HO + NO2 O + Conclusions Results O O S O O NO2 O O S O O O KIE SIE 1.54±0.02 % 0.51 +/- 0.02 O O R S O O 1.72±0.03 % CH3 • No satisfactory theoretical transition state structure yet attained • Greater than 1% KIE considered qualitatively large for sulfur BaSO4 BaCl2 BaSO4 HO Possible mechanism of acid hydrolysis of sulfate monoesters: CH3 1. Associative Mechanism: Pentavalent Intermediate O O R S O O H+ Data Acquisition2 O O R S O O H H2O O S H R O O H O H - ROH O O O H S O O H H+ O O H S O O Elemental Analyzer 2. Concerted Dissociative Mechanism: Proton Transfer in TS 18 O S O Combustion Reactor BaSO4 O O O H O 18 k nonbridge = SO2 O k = 0.02% "" values: isotopic ratio compared to a standard 0.83% (2.3%) Methodology for determination of central heavy atom isotope effects would be valuable: O O O O H O H H2O O O R S O O H R O H O O S O ROH + ROH + O H O H S O O H2O O O S HO OH O O S HO OH • A new procedure for sulfur-34 isotope effect determination is presented • S-34 KIE suggest a dissociative mechanism for acidic sulfate hydrolysis • Solvent isotope effects inconsistent with a concerted dissociative mechanism • S-34 KIE determinations may be useful for the investigation of sulfatases and sulfotransferases R O H O S O O O H H ROH + H2SO4 O H References Stable Isotope Mass Spectroscopy 3. Stepwise Dissociative Mechanisms: Preequilibrium protonation 1Hoff, R. H.; Larsen, P.; Hengge, A. C. J. Am. Chem. Soc. 2001, 123, 9338-9344. Kinetic Isotope Effect determined from delta values: O O R S O O a = RP/RSM or O S H+ H = 1000[(R - Rstd)/Rstd] S-34 KIE ? HOH O O R S O OH 15 H H O O R S O O H GC Column N H O O R S O O + Discussion BaCl2 • % completion determined by visible spectroscopy • Inorganic sulfate product precipitated with barium • Product collected at multiple points of hydrolysis k bridging = 0.97% The two mechanistic possibilities for sulfate ester hydrolysis most consistent with data: H+ O O R S O O H H2O R O H O O S O H O H ROH + O O S HO OH a= (1000+ P)/( 1000+ SM) N H •Clear up mechanistic ambiguities by giving full picture of heavy atom isotope effects •No synthetic isotopic labeling of substrate necessary •Wider range of substrates—no requirement for nitrogen in substrate O O R S O O KIE= log(1-f)/log(1-f*a) O Solvent Isotope Effects O O S O O O O S O O NO2 1 N DCl HSO4- + HO NO2 D2O NO2 1 N HCl HSO4- H+ O O R S O O H O ROH + S O O H2O HO Distinguishing between mechanisms: NO2 H2O Rate determined via visible spectroscopy Jager, H.-J.; Norman, A. L.; Krouse, H. R.; Brand, W. A. Anal. Chem. 1994, 66, 2816-2819. O O S HO OH – Central atom KIE consistent with dissociative mechanism + 2Giesemann, A.; – Inverse SIE suggests no proton transfer in transition state – Of listed mechanisms, stepwise dissociative mechanism most consistent with data Acknowledgments This work has been funded by NIH/NCI grant RO1CA71736 (TSW) and NSF grant EAR-978267 (LMP). We would like to thank Alvan Hengge for helpful discussions.