duk06_31.ppt (242Kb)
advertisement
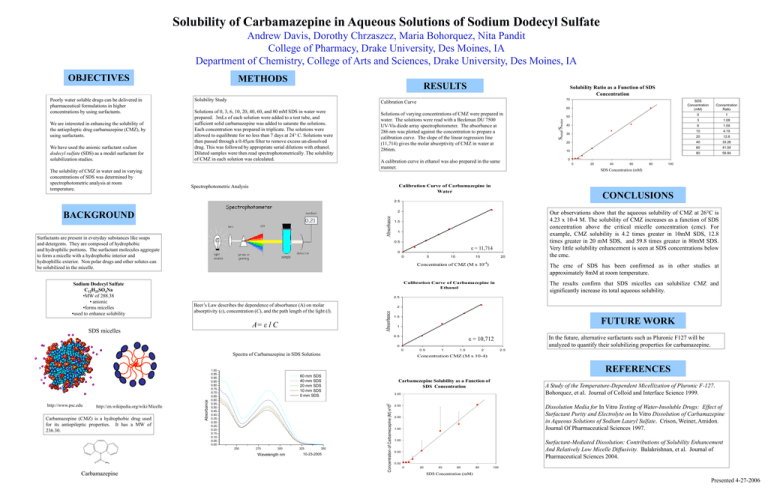
Solubility of Carbamazepine in Aqueous Solutions of Sodium Dodecyl Sulfate Andrew Davis, Dorothy Chrzaszcz, Maria Bohorquez, Nita Pandit College of Pharmacy, Drake University, Des Moines, IA Department of Chemistry, College of Arts and Sciences, Drake University, Des Moines, IA Poorly water soluble drugs can be delivered in pharmaceutical formulations in higher concentrations by using surfactants. We are interested in enhancing the solubility of the antiepileptic drug carbamazepine (CMZ), by using surfactants. We have used the anionic surfactant sodium dodecyl sulfate (SDS) as a model surfactant for solubilization studies. The solubility of CMZ in water and in varying concentrations of SDS was determined by spectrophotometric analysis at room temperature. METHODS RESULTS Solubility Study Solubility Ratio as a Function of SDS Concentration 70 Calibration Curve Solutions of 0, 3, 6, 10, 20, 40, 60, and 80 mM SDS in water were prepared. 3mLs of each solution were added to a test tube, and sufficient solid carbamazepine was added to saturate the solutions. Each concentration was prepared in triplicate. The solutions were allowed to equilibrate for no less than 7 days at 24° C. Solutions were then passed through a 0.45µm filter to remove excess un-dissolved drug. This was followed by appropriate serial dilutions with ethanol. Diluted samples were then read spectrophotometrically. The solubility of CMZ in each solution was calculated. 60 Solutions of varying concentrations of CMZ were prepared in water. The solutions were read with a Beckman DU 7500 UV-Vis diode array spectrophotometer. The absorbance at 286 nm was plotted against the concentration to prepare a calibration curve. The slope of the linear regression line (11,714) gives the molar absorptivity of CMZ in water at 286nm. Absorbance 1 0.5 ε = 11,714 5 10 15 20 Concentration of CMZ (M x 10-4) Beer’s Law describes the dependence of absorbance (A) on molar absorptivity (ε), concentration (C), and the path length of the light (l). A= ε l C 1.5 1.09 10 4.19 20 12.8 40 33.26 60 41.04 80 59.84 20 40 60 80 100 Our observations show that the aqueous solubility of CMZ at 26°C is 4.23 x 10-4 M. The solubility of CMZ increases as a function of SDS concentration above the critical micelle concentration (cmc). For example, CMZ solubility is 4.2 times greater in 10mM SDS, 12.8 times greater in 20 mM SDS, and 59.8 times greater in 80mM SDS. Very little solubility enhancement is seen at SDS concentrations below the cmc. FUTURE WORK 1 ε = 10,712 0 In the future, alternative surfactants such as Pluronic F127 will be analyzed to quantify their solubilizing properties for carbamazepine. 0.5 1 1.5 2 2.5 Concentration CMZ (M x 10-4) REFERENCES 1.00 0.95 0.90 0.85 0.80 0.75 0.70 0.65 0.60 0.55 0.50 0.45 0.40 0.35 0.30 0.25 0.20 0.15 0.10 0.05 0.00 60 mm SDS 40 mm SDS 20 mm SDS 10 mm SDS 0 mm SDS Carbamezepine Solubility as a Function of SDS Concentration -2 250 275 300 Wavelength nm 325 350 10-23-2005 A Study of the Temperature-Dependent Micellization of Pluronic F-127. Bohorquez, et al. Journal of Colloid and Interface Science 1999. 3.00 Concentration of Carbamezapine (M) x10 Absorbance Spectra of Carbamazepine in SDS Solutions Carbamazepine 6 The results confirm that SDS micelles can solubilize CMZ and significantly increase its total aqueous solubility. 0 Carbamazepine (CMZ) is a hydrophobic drug used for its antiepileptic properties. It has a MW of 236.30. 1.08 2 0.5 http://en.wikipedia.org/wiki/Micelle 3 2.5 SDS micelles http://www.psc.edu 1 The cmc of SDS has been confirmed as in other studies at approximately 8mM at room temperature. Calibration Curve of Carbamazepine in Ethanol Abssorbance Sodium Dodecyl Sulfate C12H25SO4Na •MW of 288.38 • anionic •forms micelles •used to enhance solubility 1.5 0 10 0 CONCLUSIONS 2 0 20 Concentration Ratio SDS Concentration (mM) 2.5 Surfactants are present in everyday substances like soaps and detergents. They are composed of hydrophobic and hydrophilic portions. The surfactant molecules aggregate to form a micelle with a hydrophobic interior and hydrophillic exterior. Non polar drugs and other solutes can be solubilized in the micelle. 30 0 Calibration Curve of Carbamazepine in Water BACKGROUND 40 SDS Concentration (mM) 0 A calibration curve in ethanol was also prepared in the same manner. Spectrophotometric Analysis 50 Stotal/Swater OBJECTIVES 2.50 Dissolution Media for In Vitro Testing of Water-Insoluble Drugs: Effect of Surfactant Purity and Electrolyte on In Vitro Dissolution of Carbamazepine in Aqueous Solutions of Sodium Lauryl Sulfate. Crison, Weiner, Amidon. Journal Of Pharmaceutical Sciences 1997. 2.00 1.50 1.00 Surfactant-Mediated Dissolution: Contributions of Solubility Enhancement And Relatively Low Micelle Diffusivity. Balakrishnan, et al. Journal of Pharmaceutical Sciences 2004. 0.50 0.00 0 20 40 60 80 100 SDS Concentration (mM) Presented 4-27-2006




