Lecture 3 Boiling Points
advertisement

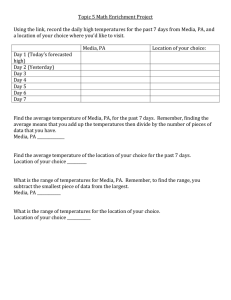
Adventures in Thermochemistry James S. Chickos* Department of Chemistry and Biochemistry University of Missouri-St. Louis Louis MO 63121 E-mail: jsc@umsl.edu Eads Bridge 1867-72 6 Based on the behavior observed in the melting temperatures of homologous series, we wondered how boiling temperatures varied as a function of size? Question: How do the boiling temperatures of the n-alkanes vary as a function of the number of repeat units? 800 700 600 TB 500 The plot of the boiling temperatures of the n-alkanes as a function of the number of repeat units. 400 300 200 0 5 10 15 Number of repeat units, n 20 25 Modeling boiling temperature Exponential functions have previously been used to model the behavior observed for the n-alkanes. TB (M ) TB () α * e bM 2 / 3 1 M = molecular weight; , b = constants TB = 138 C1/2; C = number of carbons 2 Is there any basis for expecting the boiling temperature of an infinite alkane to be finite? 1. Kreglewski, A.; Zwolinski, B. J. J. Phys. Chem. 1961 65, 1050-1052. 2. Partington, J. An Advanced Treatise on Physical Chemistry, Vol II, Properties of Liquids, Longmans, Green Co.: N. Y., 1949, p 301. 70000 A plot of lgHm(TB) versus lgSm(TB) at T = TB for the following: lgHm(TB) / J mol-1 60000 50000 n-alkanes (C3 to C20): circles, 40000 n-alkylcyclopentanes (C7 to C21): triangles, 30000 n-alkylcyclohexanes (C8 to C24): squares. 20000 10000 80 82 84 86 lgSm(TB) 88 90 / J mol-1 K-1 92 94 If the relationship between lgHm(TB) and lgSm(TB) can be expressed in the form of an equation of a straight line: lgHm(TB) = m lgSm(TB) + C (1) Since at the boiling temperature, lgGm(TB) = 0; lgSm(TB) = lgHm(TB)/TB Therefore lgHm(TB) = m lgHm(TB)/ TB +C Solving for TB: TB = m lgHm(TB)/( lgHm(TB) - C) This is an equation of a hyperbola As lgHm(TB) ; TB m (2) The Correlation Equations Obtained by Plotting lgHm(TB) Versus lgSm(TB) n-alkanes lgHm(TB) = (3190.722.6) lgSm(TB) – (240583350); r2 = 0.9992 n 1-alkenes lgHm(TB) = (2469.3109.7) lgSm(TB) – (169585951); r2 = 0.9806 n-alkylbenzenes lgHm(TB) = (3370.537.3) lgSm(TB) – (247175296); r2 = 0.9985 n-alkylcyclopentanes lgHm(TB) = (3028.897.4) lgSm(TB) – (220567926); r2 = 0.9877 n-alkylcyclohexanes lgHm(TB) = (3717.887.3) lgSm(TB) – (284890999); r2 = 0.9918 n-alkanethiols lgHm(TB) = (2268.7162.6) lgSm(TB) – (1616931728); TB() ~ 3000 K r2 = 0.9558 If TB approaches 3000 K in an ascending hyperbolic fashion, then a plot of 1/[1 – TB/TB()] versus n, the number of repeat units, should result in a straight line. A plot of 1/[1- TB/TB()] versus the number of methylene groups using a value of TB() = 3000 K. 1.30 1/[1 - TB/TB( 1.25 1.20 1.15 squares: phenylalkanes hexagons: alkylcyclopentanes circles: n-alkanes triangles: 1-alkenes 1.10 1.05 0 5 10 15 20 N, number of methylene groups, n 25 Use of TB() = 3000 K did not result in straight lines as expected. Therefore: TB() was treated as a variable and allowed to vary in 5 K increments until the best straight line was obtained by using a non-linear least squares program resulting in the following. 1/[1-TB(N)/TB( 2 squares: phenylalkanes hexagons: alkylcyclopentanes circles: n-alkanes triangles: 1-alkenes 1 0 5 10 15 N, number of methylene groups 1/[1- TB/TB()] = aN + b 20 The Results Obtained by Treating TB of a Series of Homologous Compounds as Function of the Number of Repeat Units, N, and Allowing TB() to Vary; aBm, bBm: Values of aB and bB Obtained by Using the Mean Value of TB() = 1217 K Using TB()avg = 1217 K /K aBm /K data points 0.06231 1.214 0.9 0.04694 1.1984 3.6 18 2-methyl-n-alkanes 1110 0.05675 1.3164 0.2 0.0461 1.2868 0.3 8 1-alkenes 0.06025 1.265 0.4 0.04655 1.242 2.7 17 n-alkylcyclopentanes 1140 0.05601 1.4369 0.6 0.04732 1.4037 1.3 15 n-alkylcyclohexanes 1120 0.05921 1.5054 0.1 0.04723 1.4543 1.2 13 n-alkylbenzenes 1140 0.05534 1.5027 1.1 0.05684 1.5074 1.4 15 1-amino-n-alkanes 1185 0.04893 1.274 3.4 0.04607 1.267 3.4 15 1-chloro-n-alkanes 1125 0.05717 1.2831 0.3 0.04775 1.2628 1.6 13 1-bromo-n-alkanes 1125 0.05740 1.3264 1.0 0.0481 1.2993 1.5 12 1-fluoro-n-alkanes 1075 0.05833 1.2214 0.4 0.04495 1.1987 2.1 9 1-hydroxy-n-alkanes 1820 0.01806 1.220 0.8 0.03953 1.3559 3.6 12 2-hydroxy-n-alkanes 1055 0.05131 1.4923 1.8 0.03732 1.4031 1.8 7 n-alkanals 910 0.08139 1.4561 1.4 0.04277 1.3177 2.5 7 1440 0.03071 1.2905 1.6 0.0430 1.3613 1.7 8 Polyethylene Series TB()/K n-alkanes 2-alkanones 1076 1090 aB bB bBm Polyethylene Series TB()/K aB bB /K aBm bBm /K data points n-alkane-1-thiols 1090 0.06170 1.3322 0.2 0.042 1.3635 2.8 14 n-dialkyl disulfides 1190 0.08720 1.4739 0.4 0.08207 1.4608 0.6 9 n-alkylnitriles 1855 0.01907 1.2294 2.6 0.04295 1.3869 3.4 11 n-alkanoic acids 1185 0.0440 1.4964 1.3 0.04100 1.4790 1.3 16 0.03158 1.3069 2.6 0.04200 1.3635 2.8 10 methyl n-alkanoates 1395 Mean Value of TB() = (1217246) K The results for TB() for polyethylene are remarkably constant considering the use of data with finite values of n to evaluate TB(n) for n (). These results are also in good agreement with the values reported previously for the n-alkanes by Kreglewski and Zwolinski (TB() = 1078 K), Somayajulu (TB() = 1021 K), Stiel and Thodos ((TB() = 1209) K. Kreglewski, A.; Zwolinski, B. J. J. Phys. Chem. 1961 65, 1050-1052. Somayajulu, G. R. Internat. J. Thermophys. 1990, 11, 555-72. Stiel, L. T.; Thodos, G. AIChE. J. 1962, 8, 527-9. A value of TB() = (1217246) K is considerably less than TB() = 3000 K, the value obtained by assuming that lgHm(TB) as TB . Why is TB() = (1217246) K, not ~3000 K? From the plot of lgHm(TB) vs lgSm(TB), shown earlier: TB = m lgHm(TB)/( lgHm(TB) - C) Rearranging and solving for lgHm(TB)max using TB() = 1217 results in: lgHm(TB)max = C (TB())/(m - TB()) lgHm(TB)max = 154.5 18.5 kJ mol-1 A limiting value of 154.5 18.5 kJ mol-1 for lgHm(TB)max at TB is predicted where C and m are from plots of lgHm(TB) vs lgHm(TB) A limiting value for lgHm(TB)max suggests that this property may also be modeled effectively by a hyperbolic function 1.8 1/[1-lgHm(TB)/lgHm(TBmax 1.7 A plot of 1/[1- lgHm(TB)/ lgHm(TB)max] against the number of repeat units, n 1.6 1.5 1-alkenes: circles 1.4 n-alkylcyclohexanes: squares 1.3 using a value of 154 kJ mol-1 for lgHm(TB)max.. 1.2 1.1 0 2 4 6 8 10 12 14 16 18 n, number of repeat units, n Data from: Wilhoit, R. C.; Zwolinski B. J. Handbook of Vapor Pressures and Heats of Vaporization of Hydrocarbons and Related Compounds. TRC, Texas A&M Univ. College Station TX Values of the Parameters of aH and bH Generated in Fitting lgHm(TB) of Several Homologous Series Using a Value of lgHm(TB)max = 154.5 18.5 kJ mol-1. aH bH /kJ.mol-1 data points n-alkanes 0.02960 1.1235 0.4 18 n-alkylbenzenes 0.02741 1.284 0.5 15 n-alkylcyclohexanes 0.02697 1.2754 0.2 15 n-alkylcyclopentanes 0.02821 1.2475 0.2 15 n-alk-1-enes 0.02796 1.1554 0.4 17 n-alkane-1-thiols 0.03172 1.1854 0.5 13 At this point it might be useful to ponder why vaporization enthalpies may approach a limiting value. Consider what vaporization enthalpies measure: intermolecular forces As the size of a flexible molecule increases, what trend would be expected in the ratio of intermolecular/intramolecular interactions? In the limiting case, for a flexible molecule the ratio between intermolecular/intramolecular interactions might be expected to go as the ratio of the surface area of a sphere to its volume: 4r2/4/3 r3 ~ 1/r Why do all of the series related to polyethylene converge to a value for lgHm(TB)max = 154.5 18.5 kJ mol-1 ? Why do all of the series related to polyethylene converge to a value for lgHm(TB)max = 154.5 18.5 kJ mol-1 ? Ambroses’ Equation TC = TB + TB/[c + d(n+2)] where c and d are constants and n refers to the number of methylene groups. This equation suggests that TC TB as n . How do critical temperatures of homologous series vary with n? Ambrose, D. "NPL Report Chemistry 92" (National Physical Laboratory, Teddington, Middlesex UK, 1978). Experimental Critical Temperatures 900 A plot of experimental critical temperatures versus n, the number of methylene groups for (from top to bottom): TC / K, Critical temperature 800 700 alkanoic acids: hexagons, 600 2-alkanones: diamonds, 1-alkanols: solid circles, 500 1-alkenes: 400 triangles, and n-alkanes: circles. 300 0 5 10 15 number of methylene groups, n 20 25 According to Ambroses’ equation and the previous plots, the critical temperatures of series related to polyethylene appear to behave in an ascending hyperbolic fashion. This suggests that a plot of 1/[1- TC /TC()] versus the number of methylene groups n should also be a linear function provided a suitable value of TC() was used. Treating TC() as a variable in ± 5 K increments, a non linear least squares fit the data resulted in the following: 5 •carboxylic acids 1/[1-Tc/Tc()] 4 2-alkanones 3 n-alkanes 2 1 0 0 5 10 15 Number of CH2 groups 20 25 Results Obtained for the Constants aC and bC by plotting 1/[1-TC(n)/ TC() as a Function of the Number of Repeat Units, N, and Allowing TC() to Vary; aCm, bCm: Values of aC and bC Obtained by Using the Mean Value of TC = 1217 K Polyethylene data Series TC()/K aC bC /K TC()/K aCm bCm /K points n-alkanes 1050 0.1292 1.4225 1.7 1217 0.07445 1.4029 9.8 16 n-alkanals 1070 0.1171 1.7753 1.0 1217 0.07756 1.6355 1.8 8 alkanoic acids 1105 0.0961 2.1137 3.4 1217 0.06456 1.9329 3.9 31 1-alkanols 1045 0.1157 1.8362 3.6 1217 0.06773 1.6639 4.7 11 2-alkanones 1105 0.10063 1.8371 1.3 1217 0.07193 1.718 1.9 11 3-alkanones 1185 0.07827 1.8168 1.3 1217 0.07158 1.7811 1.3 10 1-alkenes 1035 0.1327 1.5496 0.3 1217 0.08278 1.4518 3.1 8 2-methylalkanes 950 0.16282 1.7767 0.6 1217 0.07862 1.5329 1.7 5 Critical Temperatures vs n 900 TC / K, Critical temperature 800 A plot of experimental critical temperatures versus n, the number of methylene groups for (from top to bottom): alkanoic acids: hexagons, 700 2-alkanones: diamonds, 600 1-alkanols: solid circles, 500 1-alkenes: triangles, 400 and n-alkanes: circles. 300 The lines were calculated using TC() = 1217 K. 25 0 5 10 15 number of methylene groups, n 20 What are the consequences if TB () = TC ()? What are the consequences if TB () = TC ()? At TC, lgHm(TC) = 0 This explains why lgHm(TB) fails to continue to increase but may infact decrease as the size of the molecule get larger. What does lgHm(TB) measure? If vaporization enthalpies are a measure of intermolecular interactions, as the size of the molecule get larger, the ratio of intermolecular/intramolecular interactions 0 as n . Are there any additional consequences if TB () = TC ()? Since TB is the normal boiling temperature, If TC () = TB (), then in the limit, PC () = PB () = 101.325 kPa; 0.1 MPa. The critical pressure should decrease with increasing n asympotically approaching 0.1MPa as n . Therefore a plot of 1/[1- PC()/PC (n)] versus n using PC() = 0.1 MPa should result in a straight line. 1/[1-Pc () /Pc] vs n 1.09 A Plot of 1.08 1/[1-Pc () /Pc] vs n 1/[1-Pc/Pc()] 1.07 for carboxylic acids 1.06 1.05 1.04 1.03 1.02 1.01 0 2 4 6 8 10 12 n, number of CH2 groups 14 16 18 Critical Pressures vs n A. plot of the critical pressure versus the number of repeat units for the 1-alkanols: triangles, n-alkanes: circles, 2-methylalkanes: squares 7 6 PC / MPa 5 4 3 2 1 0 0 5 10 15 number of repeat units, n 20 25 What about other series? What about other series? How about the fluorocarbons? Boiling Temperatures Versus the Number of CF2 Groups TB /K 600 550 symbols: experimental TB / K 500 lines: 450 calculated TB / K circles: prefluoroalkanes 400 350 squares: 300 perfluorocarboxylic acids 250 200 0 2 4 6 8 10 12 n, number of CF2 groups 14 16 Table 7. Values of the Parameters of aB and bB Generated in Fitting TB of Several Homologous Perfluorinated Series Using Equation 3 and Allowing TB() to Vary in 5 K Increments; aBm, bBm: Values of aB and bB Using an Average Value of TB() = 915 K TB = TB()[1-1/(1-aBN + bB)] (3) TB()/K aB bB /K TB()/K aBm bBm /K N n-perfluoroalkanes 880 0.07679 1.2905 2.1 915 0.06965 1.2816 2.2 13 915 0.07053 1.6085 1.3 8 n-perfluoroalkanoic acids 950 0.06313 1.5765 1.2 methyl n-perfluoroalkanoates 915 0.06637 1.5000 1.6 4 915 0.07409 1.3751 1.8 5 1-iodo-n-perfluoroalkanes Critical Temperatures Versus the Number of CF2 Groups 600 symbols: experimental TC / K 550 lines: 500 calculated TC / K 450 using TC = 915 K 400 for the nperfluoroalkanoic acids 350 300 0 1 2 3 4 5 6 7 n, number of CF2 groups 8 9 Perfluoroalkanes 4 A plot of the critical pressure versus the number of repeat units using PC () = 0.101 (MPa) 3 2 1 0 0 1 2 3 4 5 6 7 8 n, number of CF2 groups 9 Conclusions: 1. Boiling temperatures appear to converge to a finite limit. 2. Vaporization enthalpies are predicted to approach a limiting value and then decrease as the size of the homologous series increases. 3. Critical temperature and boiling temperatures appear to converge as a function of the number of repeat units. 4. Critical pressures appear to converge to some finite pressure (~1 atm) as the number of repeat units . Can any of this be experimentally verified?