Lecture 1 Fusion
advertisement

Adventures in Thermochemistry
James S. Chickos*
Department of Chemistry and Biochemistry
University of Missouri-St. Louis
Louis MO 63121
E-mail: jsc@umsl.edu
A portion of the Science
Complex UMSL
Adventures in Thermochemistry
Research Interests
Phase Transitions and Their Properties
Fusion Enthalpies
Vaporization Enthalpies
Sublimation Enthalpies
Estimating vaporization enthalpies
Estimating fusion enthalpies
Estimating melting temperatures
Estimating boiling temperatures
Evaluating vapor pressures (liquid and solid)
Estimating heat capacities (solid and liquid)
Hypothetical Thermodynamic Properties
Phase change properties have been measured and studied for over
200 years. Many methods have been developed for measuring and
estimated these properties. One of the major interests in my
research group was focused on developing methods both
experimental and computational that would allow studies of the
properties of materials that can not measured directly because of
their characteristics. For example, they decompose at the
temperatures needed for measurement, they exist naturally as
mixtures, or the property is experimentally inaccessible.
Hypothetical thermodynamic properties: useful in constructing
thermochemical cycles
Vapor pressures of many solid polutants are relatively non-volatile and
present in very low concentrations on particulate matter. The vapor
pressures of these materials can be modeled by the vapor pressure of
the sub-cooled liquid.
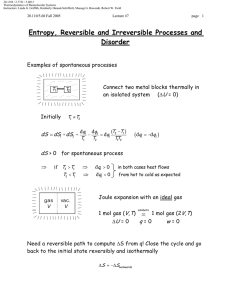
Fusion Enthalpies
1. Measurement
2. Their estimation
Measurements of Fusion Enthalpies
Perkin Elmer DSC -7
Estimation of Thermodynamic Properties
A measurement always results in a number. Estimations though
only approximate offer a rational way of deciding on whether the
result is reasonable or not. This is particularly useful to those of us
that are training students.
Estimation of Fusion Enthalpies
The direct estimation of fusion enthalpies is problematic for the
following :
∆Htrns(226 K)
The DSC heating curve of CCl4:
4.58 kJmol-1
Heat Flow Endo UP mW
45
40
∆Hfus(251 K)
35
2.52 kJmol-1
30
25
20
-80
-60
-40
T/°C
-20
0
Fusion enthalpy does not seem amenable to a group additivity approach;
neither does fusion entropy
Waldon’s Rule: ∆Hfus(Tfus)/Tfus ≈ 54.4 Jmol-1K-1
∆Hfus(Tfus)
dodecane
tridecane
tetradecane
pentadecane
36820
28490
7660
45070
34600
9170
Tfus
263.6
267.8
255
279
283.1
270.9
∆Sfus(Tfus)
139.7
106.3
30.0
161.5
122.2
33.9
∆Stpce
139.7
136.3
165.5
156.1
Consider however the total phase change entropy from T = (0 to Tfus) K.
Is total phase change entropy (∆Stpce) treatable by group additivity?
Our Philosophy Regarding Estimation Methods
There seem to be two philosophies regarding estimation methods
including group additivity
1. Devise a simple and consequently approximate method
2. Devise a method that is as precise as possible
Our experience has been that
the simplest method wins
out. It is used the most and
misused the least. Methods
that have numerous
parameters are often
improperly used and
sometimes these parameters
are simply parameterizing
experimental error.
Estimating total Phase Change Entropy
b
b
b
. b Used with function groups attached.
Some Simple Estimations
∆Stpce = 1.31*7.1*nCH2 + 17.6*nCH3
Alkanes
dodecane
tridecane
tetradecane
pentadecane
tridecane
∆Hfus(Tfus)
Jmol-1
Tfus/K
36820
28490
45070
34600
263.6
267.8
279
283.1
∆Stpce (exp) ∆Stpce (est) ∆Htpce(Tfus)est
Jmol-1K-1
Jmol-1
139.7
136.4
161.5
158.0
128
138
147
156
33740
36960
41000
44160
∆Stpce (exp) = 28490/267.8 + 7660/255 = 136.4 Jmol-1K-1
∆Htpce (exp) = 28490 + 7660 = 36150 Jmol-1
pentadecane ∆Stpce (exp) = 34600/283.1 + 9170/270.9 = 158.0 Jmol-1K-1
∆Htpce(exp) = 34600 + 9170 = 43770 Jmol-1
Some Simple Estimations / Jmol-1
Aromatics ∆Stpce = [7.4]·n=CH- + [- 7.5] ·n=CR- + [-9.6]·n=CR’- +
[17.6]·nCH3
R = sp2 atom; R’ =sp3 atom
1-methylnaphthalene [7.4]·7+[-7.5]·2 + [-9.6] +[17.6] = 44.9 (49.3)expt
2-methylnaphthalene
= 44.9 (58.9)expt
1-methylnaphthalene ∆Htpce(Tfus)exp = 11930; ∆Htpce(Tfus)calc = 10900
2-methylnaphthalene ∆Htpce(Tfus)exp = 17740; ∆Htpce(Tfus)calc = 13800
R = any atom
Tt = 116, 363, 262 K
∆Ht = 1.4, 7.0, 11.0
∆Htpce = 12.36
∆Stpce = 37.8
∆Stpce = [33.4] + [3.7]·2 –[12.3]·3 – [1.6]·2
+ [7.4]·6 – [7.5] = 37.8
∆Hfus(Tfus) = 11.0 kJmol-1 exp
∆Htpce(Tfus) = 13.6 calc
= 48.0
= 22.1 (exp0
∆Htpce(Tfus) = 23.5 calc
= 45.4
∆Hfus(Tfus) = 8.55 exp
C14H12ClNO 2
Tolenamic Acid
CH 3
Cl
CO 2H
NH
7(=CH-) a + 4(=CR'-) a + 1(=CR-) a + 1(CH 3-)
+ 1(Cl-) + 1(-CO2H) + 1(-NH-)
7(7.4) + 4(-7.5) + 1(-9.6) + 1(17.6)
+ (10.8)(1.) + 1(13.4)(2.25) + 1(-5.3)
Calc
∆Sfus(Tfus) =
80.5;
∆Hfus(484.2; 485.8 K) = 38980; 39100
Exp
∆Sfus(Tfus) =
84.7; 100.9
∆Hfus(484.2; 485.8 K) = 41000; 49000
two polymorphs
Values in [ ] are tentative assignments
C16H14O6 Hespiritin
OCH 3
HO
O
OH
OH
O
[33.4] + {3.7][n-3] + [O] c + [C=O] c + 2[=C-R] c +
[-C(H)(C)(O)] c
+ 5(=CH-) a + 4(=C-R') a+ 3(HO-) + (-O-) + (CH 3-)
+ 3.7[3]
(1.2) +
(-1.4) + 2*(-34.6)
+ (-1.6)(1.92)
[33.4][33.4]
+3.7[3]
+ +[1.2]
+[-1.4]
+ [-14.7]
+ 2[-12.3]
+ 5(7.4)
+ 4(-7.5)
3(1.7)(13.1)
+ (4.71)+3[20.3]
+ (17.5) +[4.7]
+ [17.6]
+ 5[7.4]
+ +[-9.6]
+ 4[-7.5]
Calc
∆Sfus(Tfus) = 85.6
∆Hfus(499.2 K) = 42.7
Exp
∆Sfus(Tfus) = 71.9
∆Hfus(499.2 K) = 35.9
Why do some molecules have errors
greater than ±3 ?
A series of compounds
forming liquid crystals
∆Stpce = Σ ∆Hi/Ti
On of the questions left to be answered is why
do liquid crystals behave in this way?
The total phase change entropy is not very useful unless the
fusion temperature is available. Our next adventure into
trying to predict melting temperatures resulted in some
surprises.
Reference and Acknowledgement
Total Phase Change Entropies and Enthalpies. An Update on Fusion
Enthalpies and Their Estimation. Chickos, J. S.; Acree Jr. W. E.
Thermochim. Acta 2009, 495, 5-13 and references cited.