Measurements
advertisement
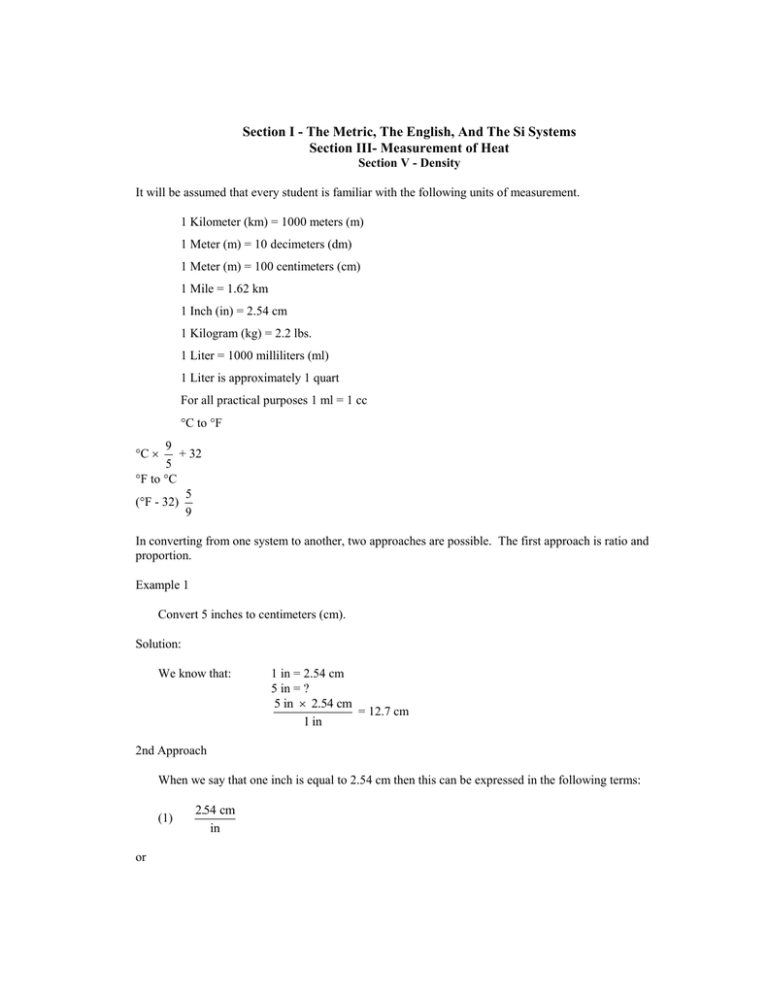
Section I - The Metric, The English, And The Si Systems Section III- Measurement of Heat Section V - Density It will be assumed that every student is familiar with the following units of measurement. 1 Kilometer (km) = 1000 meters (m) 1 Meter (m) = 10 decimeters (dm) 1 Meter (m) = 100 centimeters (cm) 1 Mile = 1.62 km 1 Inch (in) = 2.54 cm 1 Kilogram (kg) = 2.2 lbs. 1 Liter = 1000 milliliters (ml) 1 Liter is approximately 1 quart For all practical purposes 1 ml = 1 cc C to F 9 + 32 5 F to C 5 (F - 32) 9 C In converting from one system to another, two approaches are possible. The first approach is ratio and proportion. Example 1 Convert 5 inches to centimeters (cm). Solution: We know that: 1 in = 2.54 cm 5 in = ? 5 in 2.54 cm = 12.7 cm 1 in 2nd Approach When we say that one inch is equal to 2.54 cm then this can be expressed in the following terms: (1) or 2.54 cm in 1 in 2.54 cm (2) then: 2.54 cm 5 in = 12.7 cm 1 in Example 2: Convert 5 hours to seconds. Solution: 5 hrs. 60 min. 60 sec. = 18,000 sec. hr. min. Solution by ratio proportion: 1 hr. = 60 min. 5 hr. 60 min. 300 min. hr. 5 hr. = ? 1 min. = 60 sec. 300 min. 60 sec. = 18,000 sec. 1 min. 300 min. = ? Example 3: Convert 5 mg to kg. Solution: 1 g = 1000 mg ? = 5 mg 1 g = .005 g 1000 g 5 mg 1 kg = 1000 g .005 g 1 kg = .000005 kg or 5 10-6 kg 1000 g ? = .005 g The second approach is a little easier to follow: 5mg 1g 1 kg = 5 10-6 kg 1000 mg 1000 g Example 4: Convert 20C to F Solution: 20C 9 + 32 = 68F 5 Example 5: Convert 14F to C Solution: C = (F - 32) 5 9 = (14 - 32) 9 5 = (-18) 9 5 = -10C SECTION II Conversion Problems Convert: 1. 4.5 m to mm 2. 50 mg to kg 3. 105 yds. to meters 4. 50 ml to liters 5. 1 lb. to grams 6. 1 mile to cm. 7. 30C to F 8. -40C to F 9. 212F to C 10. -31F toC SECTION III MEASUREMENT OF HEAT Equation 1. Specific Heat = calories (metric) ( C)(g) Equation 2. Specific Heat = joules (SI) ( K)(g) Equation 3. Heat Energy = (mass) (specific heat) (T) Equation 4. 1 calorie = 4.184 joules Example 6: Calculate the number of calories needed to raise the temperature of 10 g of water from 5C to 20C. Solution: heat = (10g) (1 calorie) (20 - 5) C ( C)(g) = 150 calories Example 7: Calculate the number of calories needed to raise the temperature of 100 g of Fe from 10C to 50C. .11 cal . The specific heat of Fe is ( C)(g) Solution: Heat = (mass) (sp. ht.) (T) (.11 cal) = (100 g) (40C) ( C)(g) = 440 calories Example 8: 40 g of Fe at 100C were added to 80 g of water at 10C. Calculate the temperature of the mixture. 1 cal. (.11 cal) Given the sp. ht. of water is and sp. ht. of Fe . C. g ( C)(g) Solution: 100C Heat loss = Heat gained = (mass) (sp. ht.) (T) 100 - X X Heat Loss = Heat Gained (40) (.11) (100 - x) = (80) (x - 10) (1) X - 10 10C 440 - 4.4x = 80x - 800 -84.4x = -1240 X= 1240 84.4 X = 14.6C Example 9: Calculate the number of joules needed to raise the temperature of 100 g of water from 20C to 51C. (Given sp. ht. of water 4.184 J g-1 C-1) Solution: Heat = (m) (sp. ht.) (T) = (100 g) (4.184 J g-1 C-1) (31C) = 13,000 J or 1.3 x 104 J SECTION IV Energy Problems 1. How many calories are required to raise the temperature of 50 g of water from 30C to 80C? 2. How many calories are required to raise the temperature of 100 g of Fe (sp. ht. .11 cal from 20C (g)(C) to 90C? 3. The heat of combustion of coal is 6000 cal/g. How many grams are needed to raise the temperature of 500 g of water from 20C to 44C? 4. Calculate the resulting temperature when 200 g of Fe, (sp. ht. of water at 20C (sp. ht. Of water .11 cal / g at 80C are added to 200 g C 1 cal . g C 5. Calculate the increase in temperature when 1825 J are supplied to 85 g of sand (sp. ht. .787 J g -1 C1 ) 6. Calculate the amount of heat needed (in calories and in joules) to raise the temperature of 50 g of water from 20C to 75C. If this amount of heat is transferred to 1000 g of gold at 25C, what will the final temperature of the gold be? SECTION V DENSITY Units of density in the metric system is g/cm3 (g/ml) and in the SI system is kg/m3. Equation 5 D= M V Example 10 Calculate the density in g/cm3 and in the SI system if 50 g of a substance occupies a volume of 18 3 cm (ml) Solution: D= = D= M V 50g = 2.78 g/ml (metric) 18ml M (kg) V (m 3 ) 50g 1000g / kg = 18ml ml 10 6 3 m = 50 10 6 kg 18 1000m 3 = 2.78 103kg/m3 Example 9: Calculate the volume of a solid if its mass is 12.5 g and its density is 1.2 g/ml. Solution: From eq. 5 V= = M D 12,5g = 10.4 ml 12 . g / ml Example 10: How many liters will 880 g of a liquid occupy if its density is .88 g/ml? Solution: Using V as the subject of Eq. 5 V= = M D 880g .88g / ml = 1000 ml or 1 L Equation 6: Sp. Gr. = density of a substance density of water SECTION VI Density Problems 1. Calculate the mass of a solid if its volume is 20.5 ml and its density is 11.2 g/ml. 2. The density of a liquid is 1.2 g/ml, calculate the mass of .7 liter. 3. The density of a solution is 1.22 g/ml, calculate the volume of the solution if its mass is 1830 g. 4. 700 ml of a liquid weighs 840 g. Calculate the sp. gr. of the liquid. 5. The sp. gr. of chloroform is 1.49. Calculate the mass of 110 ml of the liquid. 6. A substance that weighs 88 g has a volume of 64 cm3. Calculate the density in g/cm3 and in kg/m3. ANSWERS TO SECTION II 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 4500 mm .00005 kg or 5 10-5 kg 96 m .05 liters 454.6 g 160934.4 cm or 1609.3 m 86F -40F 100C -35C 1. 2. 3. 4. 5. 6. ANSWERS TO SECTION IV 1. 2. 3. 4. 5. 6. 2500 cal. 770 cal. 2g 26C 27.3C or 27.3K a. 2750 cal., 11506 J ANSWERS TO SECTION VI b. 113C 229.6 g 840 g 1.5 or 1500 ml 1.2 163.9 g 1.4 g/cm3 1.4 103 kg/m3





