LIPID METABOLISM
advertisement
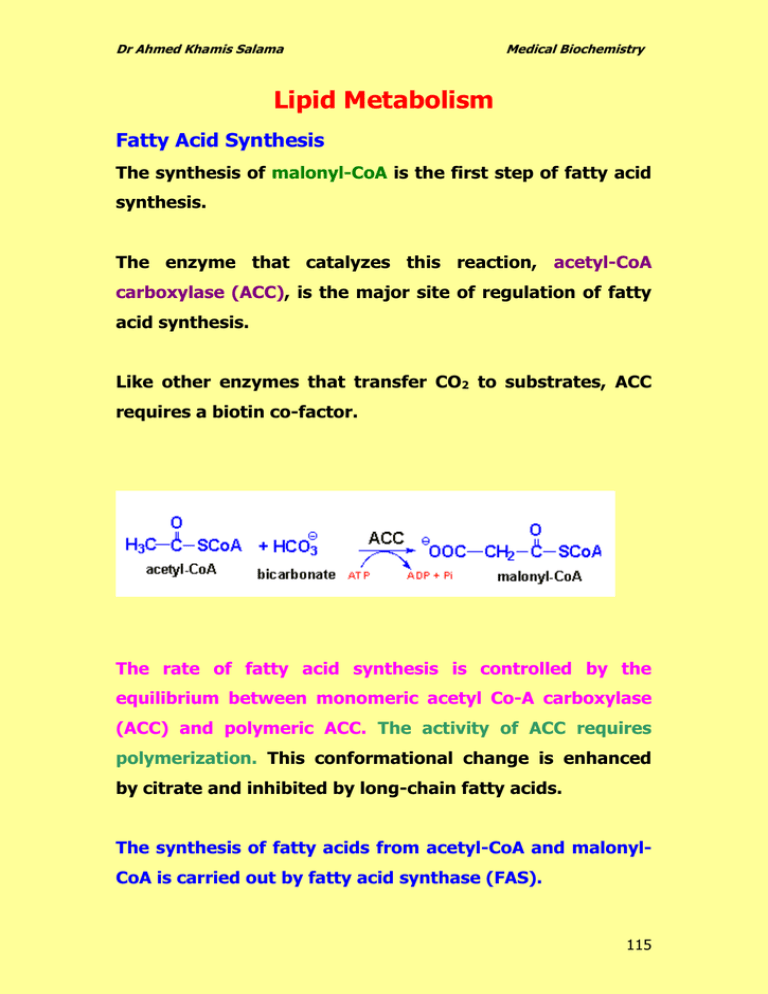
Dr Ahmed Khamis Salama Medical Biochemistry Lipid Metabolism Fatty Acid Synthesis The synthesis of malonyl-CoA is the first step of fatty acid synthesis. The enzyme that catalyzes this reaction, acetyl-CoA carboxylase (ACC), is the major site of regulation of fatty acid synthesis. Like other enzymes that transfer CO2 to substrates, ACC requires a biotin co-factor. The rate of fatty acid synthesis is controlled by the equilibrium between monomeric acetyl Co-A carboxylase (ACC) and polymeric ACC. The activity of ACC requires polymerization. This conformational change is enhanced by citrate and inhibited by long-chain fatty acids. The synthesis of fatty acids from acetyl-CoA and malonylCoA is carried out by fatty acid synthase (FAS). 115 Dr Ahmed Khamis Salama Medical Biochemistry The primary fatty acid synthesized by fatty acid synthase (FAS) is palmitate CH3 (CH2)14 COO- . Palmitate is then released from the enzyme and can then undergo separate elongation ةةةة االطالةةةة لةةةةة الكركةةةةر ال and/or unsaturation to yield other fatty acid molecules. Origin of Cytoplasmic Acetyl-CoA Acetyl-CoA is generated in the mitochondria primarily from two sources: 1. The pyruvate dehydrogenase (PDH) reaction 2. Fatty acid oxidation In order for these acetyl units to be utilized for fatty acid synthesis they must be present in the cytoplasm. Acetyl-CoA enters the cytoplasm in the form of citrate via the TCA cycle ( see the figure). In the cytoplasm, citrate is converted to oxaloacetate and acetyl-CoA by the ATP driven ATP-citrate lyase reaction. The resultant oxaloacetate is converted to malate by malate dehydrogenase (MDH). The malate produced by this pathway can undergo oxidative decarboxylation by malic enzyme to give pyruvate. The co-enzyme for this reaction is NADP+ generating NADPH. 116 Dr Ahmed Khamis Salama Medical Biochemistry The advantage of this series of reactions for converting mitochondrial acetyl-CoA into cytoplasmic acetyl-CoA is that the NADPH produced by the malic enzyme reaction can be a major source of reducing co-factor for the fatty acid synthase activities. Pathway for the movement of acetyl-CoA units from the mitochondrion to the cytoplasm for use in lipid and cholesterol biosynthesis. 117 Dr Ahmed Khamis Salama Medical Biochemistry Synthesis of Triglycerides Fatty acids are stored for future use as triglycerides in all cells, but primarily in adipocytes of adipose tissue. Triglycerides constitute molecules of glycerol to which three fatty acids have been esterified. The fatty acids present in triglycerides are predominantly saturated. The major building block for the synthesis of triglycerides, in tissues other than adipose tissue, is glycerol. Adipocytes lack glycerol kinase, therefore, dihydroxyacetone phosphate (DHAP), produced during glycolysis, is the precursor for triglyceride synthesis in adipose tissue. This means that adipoctes must have glucose to oxidize in order to store fatty acids in the form of triglycerides. Dihydroxyacetone phosphate (DHAP) can also serve as a backbone precursor for triglyceride synthesis in tissues other than adipose, but does so to a much lesser extent than glycerol. 118 Dr Ahmed Khamis Salama Medical Biochemistry Triglycerides, as major components of very low density lipoprotein (VLDL) and chylomicrons, play an important role in metabolism as energy sources and transporters of dietary fat. 119 Dr Ahmed Khamis Salama Medical Biochemistry They contain more than twice as much energy (9 kcal/g) as carbohydrates and proteins. In the intestine, triglycerides are split into monoacylglycerol and free fatty acids in a process called lipolysis, with the secretion of lipases and bile, which are subsequently moved to absorptive enterocytes, cells lining the intestines. The triglycerides are rebuilt in the enterocytes from their fragments and packaged together with cholesterol and proteins to form chylomicrons. These are excreted from the cells and collected by the lymph system and transported to the large vessels near the heart before being mixed into the blood. Various tissues can capture the chylomicrons, releasing the triglycerides to be used as a source of energy. Fat and liver cells can synthesize and store triglycerides. When the body requires fatty acids as an energy source, the hormone glucagon signals the breakdown of the triglycerides by hormone-sensitive lipase to release free fatty acids. As the brain cannot utilize fatty acids as an energy source (unless converted to a ketone), the glycerol component of triglycerides can be converted into glucose, via gluconeogenesis, for brain fuel when it is broken down. 120 Dr Ahmed Khamis Salama Medical Biochemistry Fat cells may also be broken down for that reason, if the brain's needs ever outweigh the body's. Triglycerides cannot pass through cell membranes freely. Special enzymes on the walls of blood vessels called lipoprotein lipases must break down triglycerides into free fatty acids and glycerol. Fatty acids can then be taken up by cells via the fatty acid transporter (FAT). ROLE OF TRIGLYCERIDES IN DISEASE High level of triglycerides caused Hypertriglyceridemia. In the human body, high levels of triglycerides in the bloodstream have been linked to ATHEROSCLEROSIS (thickening and loss of elasicity of arterial walls) and, by extension, the risk of heart disease and stroke. Another disease caused by high triglycerides is PANCREATITIS ياس التهاب البن. Phospholipid Structures Phospholipids are synthesized by esterification of an alcohol to the phosphate of phosphatidic acid (1,2diacylglycerol 3-phosphate). Most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone. The most commonly added alcohols (serine, ethanolamine 121 Dr Ahmed Khamis Salama Medical Biochemistry and choline) also contain nitrogen that may be positively charged, whereas, glycerol and inositol do not. The major classifications of phospholipids are: Phosphatidic acid consists of a glycerol backbone, with, in general, a saturated fatty acid bonded to carbon-1, an unsaturated fatty acid bonded to carbon-2, and a phosphate group bonded to carbon-3. Phosphatidylcholine (PC) Phosphatidylethanolamine (PE) 122 Dr Ahmed Khamis Salama Medical Biochemistry Phosphatidylserine (PS) Phosphatidylinositol (PI) Phosphatidylglycerol (PG) Diphosphatidylglycerol (DPG) 123 Dr Ahmed Khamis Salama Medical Biochemistry CHOLESTEROL METABOLISM Cholesterol is an extremely important biological molecule because it : 1. has roles in membrane structure 2. is precursor for steroid hormones synthesis 3. is precursor for the synthesis of bile acids Both dietary cholesterol and that synthesized de novo are transported through the circulation in lipoprotein particles. The same is true of cholesteryl esters, the form in which cholesterol is stored in cells. The synthesis and utilization of cholesterol must be tightly regulated in order to prevent over-accumulation and abnormal deposition within the body. Of particular importance clinically is the abnormal deposition of cholesterol and cholesterol-rich lipoproteins in the CORONARY ARTERIES. Such deposition, eventually leading to atherosclerosis, is the leading contributory factor in diseases of the coronary arteries. 124 Dr Ahmed Khamis Salama Medical Biochemistry Cholesterol Biosynthesis of Cholesterol 125 Dr Ahmed Khamis Salama Medical Biochemistry The Utilization of Cholesterol Cholesterol is transported in the plasma predominantly as cholesteryl esters associated with lipoproteins. Dietary cholesterol is transported from the small intestine to the liver within chylomicrons. Cholesterol synthesized by the liver, as well as any dietary cholesterol in the liver that exceeds hepatic needs, is transported in the serum. Cholesterol is excreted in the bile as free cholesterol or as bile salts following conversion to bile acids in the liver. Bile Acids Synthesis and Utilization The end products of cholesterol utilization are the bile acids, synthesized in the liver. Synthesis of bile acids is one of the predominant mechanisms for the excretion of excess cholesterol. However, the excretion of cholesterol in the form of bile acids is insufficient to compensate for an excess dietary intake of cholesterol. 126 Dr Ahmed Khamis Salama Medical Biochemistry Synthesis of the two primary bile acids, cholic acid and chenodeoxycholic acid. The most abundant bile acids in human bile are chenodeoxycholic acid (45%) and cholic acid (31%). These are referred to as the primary bile acids. Within the intestines the primary bile acids are acted upon by bacteria and converted to the secondary bile acids, 127 Dr Ahmed Khamis Salama Medical Biochemistry identified as deoxycholate (from cholate) and lithocholate (from chenodeoxycholate). Both primary and secondary bile acids are reabsorbed by the intestines and delivered back to the liver via the portal circulation. Clinical Significance of Bile Acid Synthesis Four physiologically functions for bile acids: 1. Their synthesis and subsequent excretion in the feces represent the only significant mechanism for the elimination of excess cholesterol. 2. Bile acids and phospholipids solubilize cholesterol in the bile, thereby preventing the precipitation of cholesterol in the gallbladder. 3. They facilitate the digestion of dietary triglycerides by acting as emulsifying agents that render fats accessible to pancreatic lipases. 4. They facilitate the intestinal absorption of fatsoluble vitamins. 128