PPrnd3
advertisement

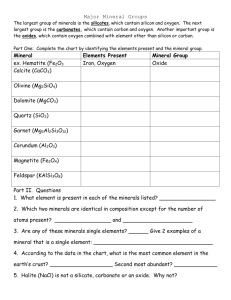
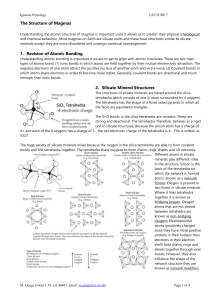
MINERAL TRIVIA FINAL ROUND! Round 3 – Question 1 Name a type of non-metal native element mineral Round 3 – Question 2 XmSn is a general formula for sulfide minerals. Name three elements that can fill in for X. Round 3 – Question 3 The spinel group of oxides has a general formula of XY2O4. Magnetite Fe3O4 is type of spinel. What type iron cations occupy the X and Y sites? Round 3 – Question 4 What is a possible anion in Halide minerals? Round 3 – Question 5 What is a common chemical feature of sulfates, carbonate, and phosphates? Round 3 – Question 6 Why is the Si-O bond in silicon tetrahedra strong? Round 3 – Question 7 What is the SixOy unit composition of sorosilicates? Round 3 – Question 8 What silicate mineral group is characterized by double chain silicon tetrahedra? Round 3 – Question 9 These pyroxenes display what feature? Round 3 – Question 10 What type of silicate is quartz which would explain its lack of cleavage? Round 3 – Question 1 Name a type of non-metal native element mineral. Sulfur, Diamond, Graphite Round 3 – Question 2 XmSn is a general formula for sulfide minerals. Name three elements that can fill in for X. Fe, Cu, Pb, Zn, Ni, ... Round 3 – Question 3 The spinel group of oxides has a general formula of XY2O4. Magnetite Fe3O4 is type of spinel. What type iron cations occupy the X and Y sites? Fe+2 in X site; Fe+3 in Y site Round 3 – Question 4 What is a possible anion in Halide minerals? Cl, F, I, or Br Round 3 – Question 5 What is a common chemical feature of sulfates, carbonate, and phosphates? All contain anionic complexes Round 3 – Question 6 Why is the Si-O bond in silicon tetrahedra strong? 50-50 ionic-covalent bond, or electrostatic valence (e.v.) = 4/4, highest in silicate minerals Round 3 – Question 7 What is the SixOy unit composition of sorosilicates? Si2O7 Round 3 – Question 8 What silicate mineral group is characterized by double chain silicon tetrahedra? Amphibole Round 3 – Question 9 These pyroxenes display what feature? Exsolution Round 3 – Question 10 What type of silicate is quartz which would explain its lack of cleavage? Tectosilicate or framework silicate And the winners are...