pH and Logarithms.doc
advertisement

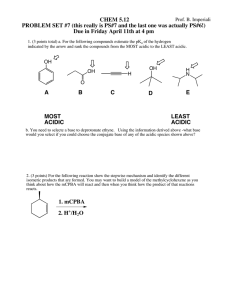
pH and Logarithms The pH Scale The acidity or alkalinity of a solution is measured by its pH, which is given by the following formula: pH log H where H measures the concentration of hydrogen ions in moles per liter. The pH scale ranges from 0 to 14. The pH of pure water is 7 which is considered neutral. A solution is considered acidic if the pH is below 7 and alkaline if its pH is above 7. Example: The pH of watermelon is 5.5, and the pH of some varieties of honey is 4.0. Which is more acidic? How many times more acidic? Compare the [H+] values. Watermelon: pH log H 5.5 logH 5.5 logH 10 H 5 .5 Honey: pH logH 4 logH 4 logH 104 H 10 4 10 5.5 104 101.5 31.6227766 To see how much, we divide 5.5 10 So lemons are about 32 times as acidic as watermelons. Example: The pH of yogurt is 4.5, while the pH of lemon juice is 4.0. Find the concentration of hydrogen ions in each food and use it to tell how many times more acidic lemon juice is than yogurt? Remember that the units of [H+] are moles per liter, M. Show all your work. Yogurt pH = -log[H+] 4.5 = -log[H+] -4.5 = log[H+] 10-4.5 = [H+] Lemon juice pH = -log[H+] 2.0 = -log[H+] -2.0 = log[H+] 10-2.0 = [H+] Since 10-2.0 > 10-4.5, divide 10-2.0 by 10-4.5. 102.0 2.0 4.5 10 104.5 102.5 316.2... Thus, lemon juice is about three hundred and sixteen times as acid as yogurt. Practice Problems 1. The pH of ibuprofen is 5.3, while the pH of Tylenol is 4.0. Which medication is more acidic? How many times more acidic is it? Remember that the units of [H+] are moles per liter, M. Show all your work. 2. The pH of tofu is 7.2, while the pH of grapefruit juice is 3.0. Find the concentration of hydrogen ions in each food and use it to tell how many times more acidic grapefruit is than tofu? Remember that the units of [H+] are moles per liter, M. Show all your work.