IRB Third-Party Access Protocol Review Form
advertisement

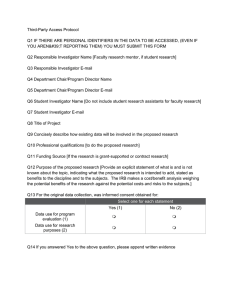
Protocol Review Form [rev March 2014] “Third Party Access to Existing Data Protocol” Protocol Number: Investigator: Title: Reviewer: A. Are the potential benefits of the study shown? B. Informed Consent/Authorization For the original data collection, was informed consent obtained for: 1. data use for program evaluation?: 2. data use for research purposes?: Yes* Yes* No No C. Is it clear what data are to be collected? Will personal identifiers be included? D. Could the research be practicably conducted without access to the data? [must be “no” for authorization] E. Could the research be practicably conducted without the waiver of authorization? [must be “no” for authorization] F. Is the research of no more than minimal risk? [must be “yes” for authorization] 1. If informed consent for use of the data for program evaluation purposes has not been established [section C1, above], researcher must show that the individuals, even if personal identifiers are absent, will not be placed at greater than minimal risk by the research [for example by substantial risk of loss of benefits depending upon the results obtained]. CSUB IRB, Third-Party Protocol Review Form, Rev March 2014 1 2. If personal identifiers are present, establish that each of the following is present: a. An adequate plan to protect identifiers from misuse; b. An adequate plan to destroy the identifiers at the earliest opportunity consistent with the research and law; c. Adequate assurances that the data will not be disclosed to any other person or entity except as required by law; d. Adequate oversight of the research project. Recommended Action: Full Approval Conditional Approval Conditions: Disapproval Signature: Date: CSUB IRB, Third-Party Protocol Review Form, Rev March 2014 2