Human Subjects Protocol, Word Version
advertisement

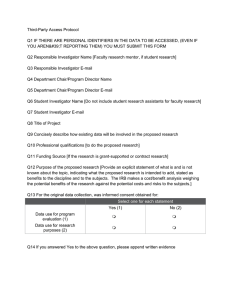
Human Subjects Protocol Q1 Human Subjects Protocol #__________________(For office use only) Protocol Q2 Please work in the MS-Word version of this document located in the Human Subjects Protocol link and then copy and paste all of the information into this form. Q3 Today's Date Q4 Select one from below for ALL new protocols not yet approved undergoing review: New Protocol (1) First Revision (2) Second Revision (3) Third Revision (4) Q5 Select one from below for modifications to previously approved protocols: First Modification (1) Second Modification (2) Third Modification (3) Q6 TITLE OF PROJECT Q7 RESPONSIBLE INVESTIGATOR NAME [Faculty research mentor, if student research.] Q8 RESPONSIBLE INVESTIGATOR E-MAIL Q9 DEPARTMENT Q10 STUDENT INVESTIGATOR NAME [Do not include student research assistants for faculty research.] Q11 STUDENT INVESTIGATOR E-MAIL ADDRESS Q12 RESEARCH ASSISTANT NAMES Q13 RESEARCH ASSISTANT E-MAIL ADDRESSES Q14 CONCISELY DESCRIBE HOW HUMAN SUBJECTS WILL BE INVOLVED IN THE PROPOSED RESEARCH Q15 PROFESSIONAL QUALIFICATIONS [to do the proposed research.] Q16 FUNDING SOURCE [if the research is grant-supported or contract research.] Q17 PURPOSE OF THE PROPOSED RESEARCH [Provide an explicit statement of what is and is not known about the topic, indicating what the proposed research is intended to add, stated as benefits to the discipline and to the subjects. The IRB makes a cost/benefit analysis weighing the potential benefits of the research against the potential costs and risks to the subjects.] Q18 METHODS [of the proposed research, including appropriateness of the design of the research. Refer to the content of the “Purpose” section as needed for clarification.] Q19 PROCEDURES [to be used, focusing on the experiences of the subjects in the research] Q20 INFORMATION SECURITY [with respect to collection, handling, storage, reporting, and destruction of research data and consent forms. Spell out the specific steps that will be taken to enhance confidentiality and protect the privacy of the subjects.] Q21 SUBJECT SELECTION CRITERIA Q22 SUBJECT EXCLUSION CRITERIA Q23 VULNERABLE POPULATIONS [Specify and justify use of subjects such as children, pregnant women, ethnic minorities, prisoners, mentally disabled persons, economically or educationally disadvantaged persons, students in the classroom, or employees in their workplace.] Q24 RISKS AND ADVERSE REACTIONS [Possible physical and emotional reactions of subjects due to participation, psychological harm and possible breaches of confidentiality. Explain precautions taken to minimize risk and how adverse reactions will be dealt with.] Q25 CIRCUMSTANCES OF INFORMED CONSENT [Address recruitment of subjects, environment or setting, time frame, condition of prospective subjects, primary language and autonomy or prospective subjects.] Q26 INFORMED CONSENT DOCUMENTED [Attach your informed consent document here] Q27 For online survey research data collection, a waiver of the requirement for written informed consent needs to be requested. Check yes below if you are requesting this waiver. Yes, see link for instructions (1) Q28 INFORMATION SHEET [Attach your Information Sheet document here] Q29 ATTACH AN ADDITIONAL DOCUMENT HERE [For example, a survey, interview schedule, authorization from an agency] Q30 ATTACH AN ADDITIONAL DOCUMENT HERE [For example, a survey, interview schedule, authorization from an agency] Q31 ATTACH AN ADDITIONAL DOCUMENT HERE [For example, a survey, interview schedule, authorization from an agency] Q32 ATTACH AN ADDITIONAL DOCUMENT HERE [For example, a survey, interview schedule, authorization from an agency] Q33 When the information in this protocol is complete, checked, approved by the faculty mentor -- if a student project -- Proceed to the submission section Q34 Required I affirm the accuracy and truth of the information in this document (1) Q35 Required I understand that submission is the first step in the IRB review and authorization process. I will receive an E-mail submission confirmation after I submit this form. (4) Last updated 12.11.2015 [Dr. Sumaya & G. Parnell]