Atomic Spectra
advertisement

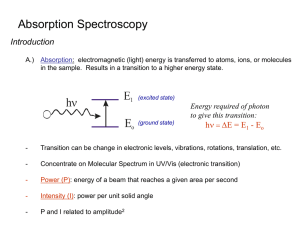
Physics Group: Raven Dean, Aime Torres, Cesar Cabato, Kathryn Morales, Jonathan Soto Faculty Advisors: Vladimir Gasparyan, Thomas Meyer Objective: We present the spectra for various elements and molecules taken with an optical spectrometer. Introduction When a low density gas is excited by an electric current, it glows, emitting light. When this light is observed through a prism or diffraction grating, it is found to consist of only a few colors, characteristic of the atoms or molecules making up the gas. Experimental Apparatus Helium 1. . . 3. 2. . Hydrogen-Balmer Series . In 1885, a Swiss high school teacher, Johann Balmer, found an empirical relationship for the wavelengths observed in 1 hydrogen : where n=2, m=3,4,5… and the Rydberg constant RH 7 -1 =1.097*10 m . Niels Bohr gave a theoretical explanation for this formula in 1913 based on the earlier discovery by Max Planck that light is quantized. The Bohr model yields a value for RH equal to the experimental result. Carbon Dioxide Water Vapor The Noble Gases . 4. Fig. 1 - Optical Spectrometer 1. Telescope 2. Collimator 3. Light Source 4. Spectrum Power Supply We use a standard optical spectroscope (Fig. 1) in which light from various sources is passed through a prism or a grating of either 300 or 600 lines per mm. We measure the angular position of the observed lines with the telescope. This allows the measurement of λ using the grating formula d sin θ = m λ, where m=1,2,3… and d is the spacing between the lines of the grating. The prism was used to show the entire emission spectra for H, He, O, N, Ne, Ar, CO2, and water vapor. All shown spectra were photographed through the telescope with an LGMS769 cell phone. For H and He we show the measured wavelength of the observed lines at the top 2 with the calculated values at the bottom (all in nm). Oxygen Neon Argon Conclusion For elements above hydrogen, many lines of characteristic wavelengths are observed, each corresponding to a specific transition of electrons between energy levels in the atom. In fact, no two elements have the same spectrum, which can be used to uniquely identify the elements present in samples of unknown content. Nitrogen References 1. Anderson, Introduction to Modern Physics, Saunders (1982) 2. http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch6/bohr.html