Episode 531: Neutron diffraction (Word, 58 KB)
advertisement
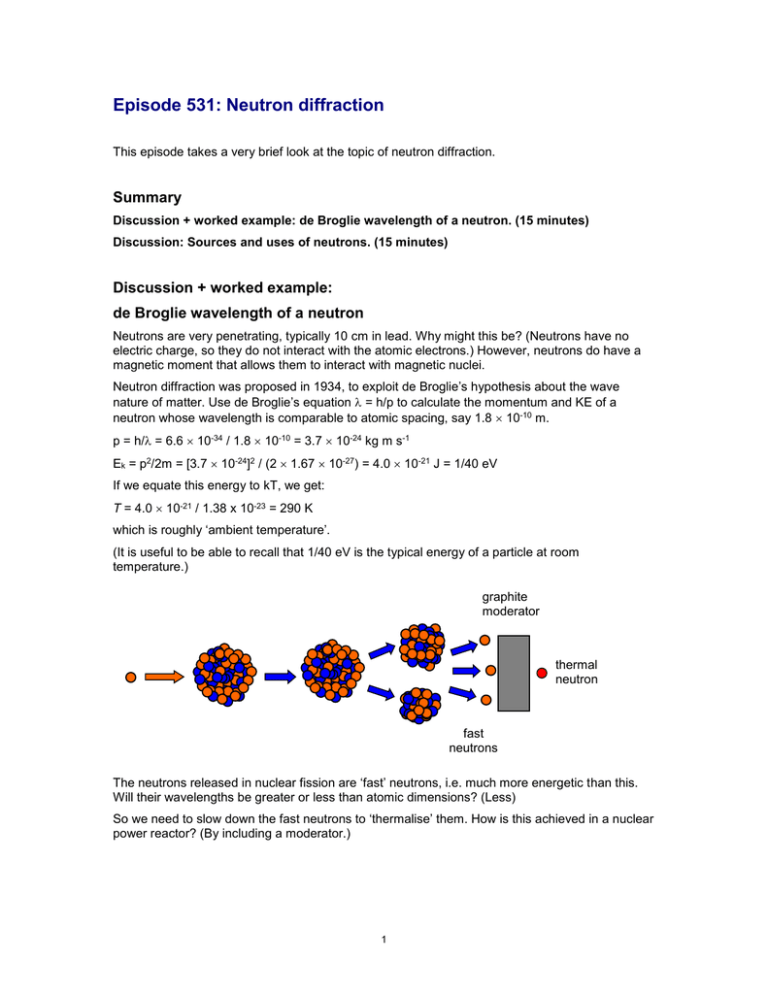
Episode 531: Neutron diffraction This episode takes a very brief look at the topic of neutron diffraction. Summary Discussion + worked example: de Broglie wavelength of a neutron. (15 minutes) Discussion: Sources and uses of neutrons. (15 minutes) Discussion + worked example: de Broglie wavelength of a neutron Neutrons are very penetrating, typically 10 cm in lead. Why might this be? (Neutrons have no electric charge, so they do not interact with the atomic electrons.) However, neutrons do have a magnetic moment that allows them to interact with magnetic nuclei. Neutron diffraction was proposed in 1934, to exploit de Broglie’s hypothesis about the wave nature of matter. Use de Broglie’s equation = h/p to calculate the momentum and KE of a neutron whose wavelength is comparable to atomic spacing, say 1.8 10-10 m. p = h/ = 6.6 10-34 / 1.8 10-10 = 3.7 10-24 kg m s-1 Ek = p2/2m = [3.7 10-24]2 / (2 1.67 10-27) = 4.0 10-21 J = 1/40 eV If we equate this energy to kT, we get: T = 4.0 10-21 / 1.38 x 10-23 = 290 K which is roughly ‘ambient temperature’. (It is useful to be able to recall that 1/40 eV is the typical energy of a particle at room temperature.) graphite moderator thermal neutron fast neutrons The neutrons released in nuclear fission are ‘fast’ neutrons, i.e. much more energetic than this. Will their wavelengths be greater or less than atomic dimensions? (Less) So we need to slow down the fast neutrons to ‘thermalise’ them. How is this achieved in a nuclear power reactor? (By including a moderator.) 1 Discussion: Sources and uses of neutrons Neutrons – where do we get them from? (Nuclear reactors; spallation sources, which use accelerators to crash ions into heavy nuclei.) What can they tell us? For elastic interactions where the neutron energy is not changed, the Bragg equation allows the determination of the separation of atomic planes. In an inelastic process, the neutron gives energy to, or receives energy from, the vibrating ions that make up the crystal lattice. This leads to information about the forces between atoms in the crystal lattice. The interaction of magnetic moments gives information about magnetic properties of materials – e.g. the position of the domain boundaries, their size and orientation, the magnetic moments of the atoms etc. 2

