Chem 1305 Syllabus Spring 2011.doc
advertisement

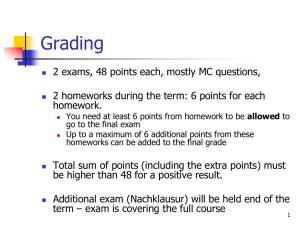
Biology & Physical Sciences Central College CHEM 1305- IntroductoryChemistry I CRN Error! Not a valid bookmark self-reference. - Spring 2011 Central College Central Campus - Room 403| 1:00- 5:00 am | Tue/Tue 4 hours Lecture, 48 hours per semester (12 Weeks) Instructor: A.C. Arami, M.S. Instructor Contact Information: E-mail: abbas.arami@hccs.edu Office location and hours LHSB 408, Tues. 12:30-1:00 Course Description CHEM 1305Introductory Chemistry Concepts & Connections, 6th edition by C.H. Corwin Prerequisites High school education or equivalent Course Goal To prepare students to take higher level Chemistry courses, or other science related courses. Student Learning Outcomes The student will be able to: 1. Apply the information learned in Chemistry to other science related courses. 2. Read and Write Chemical Formulas for simple molecules and formula units. 3. Calculate the amount of Product formed in a chemical reaction from Mass or Volume of reacting substances. 4. Determine the types of Chemical Bonds which could be formed between the elements on the Periodic Table. Learning objectives Students will: 1. Understand Uncertainties in Measurements, Significant Digits, Rounding off nonsignificant digits, Adding & Subtracting, Multiplying & Dividing Measurements, Exponential Numbers, Scientific Notation, Unit Analysis Problem Solving, & the Percent Concept, Make conversions in metric and English units, calculate volume, mass, density, temperature conversion, heat and specific heat calculations. 2. 3. Elements, Compounds, and Mixtures, Names and Symbols of the Elements, Compounds and Chemical Formulas, Physical and chemical properties & Changes, Potential and Kinetic Energy. 4. Dalton,Thomson, Rutherford, and Bohr's Model of the Atom, The Quantum Concept, Energy Levels and Sublevels, Electron Configuration, Relative Size and Shape of Atomic Orbitals 5. Classification of Elements, The Periodic Law Concept, Groups and Periods of the elements, Periodic trends, Properties of Element, Blocks of elements, Valence Electrons, Electron Dot Formulas, Ionization energy, Ionic Charges 6. Classification of Compounds, Monatomic & Polyatomic ions, Writing Chemical Formulas for: Binary Ionic, Ternary Ionic, Binary Molecular, Binary Acids, & Ternary Oxyacids, Evidences for Chemical Reactions, Writing & Balancing Chemical Equations, Classifying Chemical reactions, the Activity Series Concept, & Single Replacement Reactions, Solubility Rules & Double Displacement Reactions. Neutralization Reactions 7. Avogadro's Number, Molar mass, Molar Volume, Mole calculations, Percent Composition, Empirical Formula, Molecular Formula, Interpreting a Chemical Equation Mole-Mole Relationships, Types of Stoichiometry Problems: Mass-Mass, Mass-Volume, and Volume-Volume Problems. Limiting Reactant Concept & Limiting Reactant Problems. Percent Yield. 8. The Chemical Bond Concept, Ionic Bonds, Covalent Bonds, Electron Dot Formula of Molecules, Electron Dot Formulas of Polyatomic ions, Nonpolar Covalent Bonds, Coordinate Covalent Bonds, Hydrogen Bonds, & Shape of Molecules. Properties of Gases, Atmospheric Pressure, Gas Laws: Boyle's, Charles’s, Gay-Lussac’s, Combined, Dalton's, & Ideal Gas Law.. SCANS or Core Curriculum Statement and Other Standards Credit: 3 college Credit hours Date Chapter Classwork 1 WEEKS CALENDAR Homework 02/15 Introd.,Ch.2 Ex.2.1-2.14 Select.Ex. 02/22 Ch2 test,Ch3 Ex.3.1-3.19 Select.Ex. 03/01 Ch3 test,Ch4 Ex.4.1-4.11 Select.Ex. 03/08 Ch4 test,Ch5 Ex.5.1-5.11 Select.Ex. 03/22 Ch5 test,Ch6 Ex.6.1-6.13 Select.Ex. 03/29 Ch6 test,Ch7 Ex.7.1-7.19 Select.Ex. 04/05 Ch7 test,Ch8 Ex.8.1-8.16 Select.Ex. 04/12 Ch8 test,Ch9 Ex.9.1-9.15 Select.Ex. 04/19 Ch9 test,Ch10 Ex.10.1-10.12 Sel.Ex. 04/26 Ch10 test,Ch11 Ex.11.1-11.9 Sel.Ex. 05/03 Ch11test,Ch12 Ex.12.1-12.11 Sel.Ex. 05/10 Ch12 test & Final Exam (Ch2--Ch12) Have a good summer! FINAL EXAM Final Exam covers Ch.2 thru Ch12 of the text book. It is a comprehensive exam containing 100 multiple choice questions and problems. Instructional Methods Face to face thought provoking process which involves: solving problems, describing concepts, defining scientific terms, drawing diagrams, and explaining everything in detail on the white board using dry eraser pen. use of molecular model and over-head projector when three dimensional presentations are required. Student Assignments Final Exam Final Exam Exemption Requirements: 1.Perfect Attendance, 2. Score average of 90 or above on ch.2 thru Ch.12 tests, and 3. Completed Homeworks on all chapters. Students may not leave the room during the tests. Students arriving late for the final exam will not be allowed to take the exam if another student has already completed the Exam and left the class room. Assessments Chapter tests : 50% ( Students will be assessed weekly using 20--30 multiple choice Question / Problem test on Ch.2 thru Ch.12 of the text book ) Homework : 30% ( Students will turn in selected end of the chapter Questions and Problems after review session and before the test. Final Exam : 20% (covers Ch. 2 thru Ch. 12) Instructional Materials Text book, Calculator, White board, dry eraser pen, molecular model, over- head projector, class-size periodic table HCC Policy Statement - ADA Students who are absent more than 12 hours of class (3 sessions) will not receive credit for the course. HCC Policy Statement: Academic Honesty Students earn grades and credit for the course by use of their own knowledge. Cheating on a test includes: Copying from other students, allowing other students to copy from you, use of cell phone or an electronic device which has access to Internet. Plagiarism To steal and pass off (the ideas or words of another) as one's own, and without crediting the source.. Collusion Secret agreement or cooperation for an illegal or deceitful purpose HCC Policy Statements Any student with a documented disability (e.g., physical, learning, psychiatric, vision, hearing, etc.) who needs to arrange reasonable accommodations must contact the Disability Services office at HCC central LHSB room106 to make necessary arrangements at the beginning of each semester. HCC Course Withdrawal Policy To drop the course, it is student's responsibility to complete a” Withdrawal form", and turn it in to the Science office. Instructors will Not award a "W" to the students who will stop attending the class. Final Date for Student Withdrawal this semester is April 21 Monday Before 4:30 PM.. Repeat Course Fee If a student repeat a course three or more times he/she may face significant tuition/fee increases at HCC and other Texas Public colleges and universities. Classroom Behavior Class sessions are from 1:00 PM-- 5:00 PM Minutes will be deducted for arriving late or leaving early on the attendance roster. No Talking / Tutoring are allowed when class is in session. If you have a question you need to address it to the Instructor. Cell phones must be kept turned off during the class. Use of lap top computer is prohibited during the class. Use of Camera and/or Recording Devices Use of camera and /or recording device is prohibited without prior permission of the instructor. Instructor Requirements As your Instructor, it is my responsibility to: To explain the concepts and show how to work the problems in detail in Chapters2 through Chapter 12. It is also my responsibility to answer your chemistry questions, grade your Homeworks, grade your Tests, grade your Final Exams, average your Grades and record your Absences and Tries on the attendance roster. To be successful in this class, it is the student’s responsibility to: Plan to study 2 hours for each hour of Lecture. Come to Class Every Week and on Time. Bring your Text book and a calculator to class. Turn in Homework for each chapter before the chapter test. Look through the pages of a new chapter prior to lecture. Go to free Tutoring 12:00 Noon - 5:00 PM M thru F at LHSB 313, or by Chem. Faculty 24/7 at : www.hccs.askonline.net Program/Discipline Requirements Bring to class the following: Chemistry Text by C.H.Corwin, a scientific solar Calculator, and a note book. Have a perfect attendance, stay focused in the class during instruction, complete and turn in all homeworks. There are NO Make ups for missed Ch.Tests.. Grading Grading includes: grading of the Homeworks, Lab repots, Tests, and the Final Exam. Grading Scale College Grading System: A = 100 -- 90 ; B = 89 -- 80 ; C = 79 -- 70 D = 69 -- 60 ; F < 60 Grading Percentages Homeworks Ch.2 thru Ch12 : 30%, Chapter Tests: 50% Final Exam: 20%