Appendix_H_Rev7-03.doc
advertisement

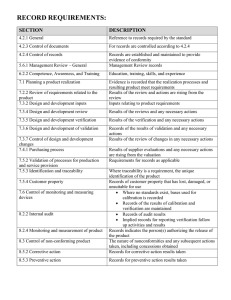
Appendix H Page 1 of 4 Procedures List [ Note: List your laboratory procedures in this section.] NCWM Publication 14, Administrative Procedures, Technical Policy, Checklist and Test Procedures No. NISTIR 7028 Administrative Procedures 62 July 2003 Appendix H Page 2 of 4 Procedures List NCWM Publication 14, Administrative Procedures, Technical Policy, Checklist and Test Procedures, Current Edition No. NISTIR 7028 Administrative Procedures 63 July 2003 Appendix H Page 3 of 4 Procedures List NCWM Publication 14, Administrative Procedures, Technical Policy, Checklist and Test Procedures No. Test Procedures and Checklists No. OIML Recommendations NISTIR 7028 64 July 2003 Appendix H Page 4 of 4 Procedures List [NOTE: The procedures in this list are those that are required by ISO/IEC 17025. The quality manual makes reference to the procedures in this list. List all the laboratory administrative procedures in this section and reference them in the appropriate sections of your quality manual. The laboratory must document and maintain these procedures as part of the laboratory quality system documentation.] Laboratory Administrative Procedures No. Procedure AP No. 1 Protection of Client Confidentiality and Proprietary Rights AP No. 2 Impartial Service AP No. 3 Document Control AP No. 4 Ensuring Traceability (includes traceability to certified reference materials, agreed methods and/or consensus standards and traceability analysis) AP No. 5 Handling Calibration and Test Items (Incoming inspection and review; Review of new incoming work; Receipt, retention, and return to include work order and work log instructions and packing and shipping instructions, avoiding deterioration, loss or damage, security) AP No. 6 Preventive Actions, Corrective Actions, Feedback AP No. 7 Internal Audits and Management Reviews (Client notification regarding adverse findings) AP No. 8 AP No. 9 Control of Data and Software Data Integrity (Security, access, verification of new software and protection and update of stored data) Purchase, Storage, and Evaluation of Supplies and Services (includes inspection and verification of quality and qualification of subcontractors) AP No. 10 Complaints AP No. 11 Laboratory Housekeeping/ Laboratory Maintenance to Support Activities and Test Results AP No. 12 Review and Maintenance of Control Charts (Covered in SOP 9, 17, 20) AP No. 13 Calibration, Verification, Maintenance, Handling, Transport, Storage, and Use of Standards AP No. 14 Calibration, Verification, Maintenance, Handling, Transport, Storage, Intermediate Calibration Status Checks, Updating Correction Status of M&TE (includes new equipment and verification of equipment outside laboratory control) AP No. 15 Departure from Documented Policies and Procedures AP No. 16 Investigation of Complaints, Adverse Audit Findings or Discrepancies, and Notifying Clients when Test Results are Affected AP No. 17 Identifying Training Needs, Training, and Qualification of Laboratory Personnel AP No. 18 Control of Non-conforming Work AP No. 19 Validation of Non-standard Test Methods to include lab designed and developed methods AP No. 20 Monitoring the Validity of Tests (Quality Control, Statistical Process Control) AP No. 21 Review of Contracts, Tenders and Work Request AP No. 22 Record Maintenance (Identification, Collection, Indexing, Access, Filing, Storage, Maintenance, and Disposal of Quality and Technical Records) AP No. 23 Sampling (Developing and Choosing Sampling Plans, Recording Relevant Data and Operations) AP No. 24 Avoiding Activities that Diminish Confidence in the Competence, Impartiality, Judgement or Operational Integrity of Tests. AP No. 25 Use of Accrediting Body Logo AP No. 26 Identifying Approved Signatories AP No. 27 Environmental Conditions for Laboratory and Field Evaluation (acceptable limits, measuring and monitoring environmental conditions, making corrections due to environmental conditions that exceed the limits ) NISTIR 7028 65 July 2003