09. WBCdisorders, hemost.doc
advertisement

D’YOUVILLE COLLEGE
BIOLOGY 307/607 - PATHOPHYSIOLOGY
Lecture 9 - BLOOD & BLOOD DISORDERS II
Chapter 7
1.
White Cell Disorders:
• leukopenia (mostly neutropenias - fig. 7 - 26 & ppt. 1):
• neutropenia - deficiency of neutrophils -- two patterns of causes
- impaired production in marrow, e.g., marrow tumor (produces non
functional cells), drugs or radiation used in tumor therapy (suppression of marrow),
other drugs (table 7 - 7), or nutrient deficiencies, e.g., vitamin B12
- excessive destruction of neutrophils, e.g., hypersplenism (entrapment in
spleen), drug-induced immune attacks, or autoimmune attacks
• leukocytosis - increased white cell levels (usually associated with
inflammations that stimulate marrow) (table 7 - 8)
- neutrophilia (response to acute inflammation) & eosinophilia (allergies or
parasitic infections) are most frequently encountered
• leukemias - tumors of leukopoietic cell lines in marrow
- neoplastic takeover of marrow results in neutropenia, anemia, bleeding
disorders due to thrombocytopenia
- invasion of adjacent bone tissue leads to bone destruction and pain in bone &
joints
- metastasis usually targets liver (hepatomegaly), spleen (splenomegaly), and
lymph nodes (lymphadenopathy) (fig. 7 - 28 & ppt. 2)
- four main types: acute myeloid leukemia (AML), acute lymphocytic
leukemia (ALL), chronic myeloid leukemia (CML), & chronic lymphocytic leukemia
(CLL); acute forms are usually more severe (fig.7 - 29 & ppt. 3)
Bio 307/607 lec 9
- p. 2 -
• lymphomas - neoplasias of lymphoid tissues that generally do not freely
circulate
Bio 307/607 lec 9
- p. 3 -
• Hodgkin's disease (lymphoma) - neoplasias that arise usually in cervical
node and follow a predictable pattern of growth and spread; four stages are
identified, based on the stage of progress through this pattern (table 7 - 9): I - single
lymph node involvement; II- several lymph nodes on same side of diaphragm; III lymph node involvement on both sides of diaphragm; IV - involvement of non
lymphoid organs
- probable cause: genetic vulnerability to Epstein-Barr virus infection
- Reed-Sternberg cells (fig. 7 - 30) are important diagnostic feature of
biopsies
- splenomegaly and lymphadenopathy are generally present as well as
depressed immune function and vulnerability to infections
• non-Hodgkin's lymphomas - also lymphoid neoplasms; growth and
spread pattern is less systematic
• bone marrow transplants: usual treatment strategy; proper matching of HLA
(MHC) between donor & recipient + suppression of recipient immune response is
required
2.
Hemostasis: prevention of blood loss
• three main steps: vascular spasm (vasoconstriction), platelet plug formation,
& coagulation
• vasoconstriction: stressed smooth muscle in wall of small artery or arteriole
responds with contraction
• platelet activation: (von Willebrand factor - factor VIII complex) - vWF-VIII
from circulating blood contacts collagen at wound site & activates platelets
- platelet plug formation: platelet activation causes aggregation to occur,
forming platelet plug (figs. 7 - 3, 7 - 8 & ppts. 4 & 5)
- platelet degranulation: platelets secrete substances (ADP, serotonin &
thromboxane) that perpetuate activation and degranulation (positive feedback),
provoke inflammation, & intensify vasoconstriction, (reduces blood loss by reducing
flow to wound site)
Bio 307/607 lec 9
- p. 4 -
- these substances collaborate with thrombin from coagulation cascade to
consolidate the clot that forms concurrently; contractile proteins in platelets
participate in clot retraction (shrinkage or consolidation of clot) (fig. 7 - 9 & ppt. 6)
- other substances from platelets assist in coagulation (platelet factor 3 PF3) and in tissue repair (platelet derived growth factor - PDGF)
Bio 307/607 lec 9
- p. 5 -
• coagulation: third step of hemostasis involves three steps of its own (in reverse
order):
- formation of fibrin from fibrinogen (plasma protein; factor I); catalyzed
by powerful clotting enzyme thrombin, which also activates fibrin stabilizing factor
(XIII), cross linking fibrin polymers to stabilize meshwork of fibrin (fig. 7 - 4 & ppt. 7)
- formation of thrombin from prothrombin (plasma protein; factor II)
- formation of prothrombin activator from reaction cascade involving
numerous additional clotting factors (mostly plasma proteins from liver {table 7 -1},
Ca2+, PF3, & tissue factor III {TF} - aka thromboplastin)
- two pathways are involved for prothrombin activator formation:
- extrinsic (triggered by TF) is faster, involving fewer steps (fig. 7 - 5 &
ppt. 8)
- intrinsic is slower, with more steps, but produces more fibrin (fig. 7 6 & ppt. 9); intrinsic pathway is triggered by thrombin, formed via extrinsic pathway
{fig. 7 - 7 & ppt. 10}, & assisted by factor VII, or alternatively triggered by Hageman
factor {XII})
- recall that Hageman factor is involved in plasma-derived mediation of
inflammation (fig. 2 - 16 & ppt. 11)
- thrombin is virtually ubiquitous in its stimulatory effects in
coagulation cascade (fibrin formation, autocatalysis of thrombin activation, activation
of platelets, & stimulation of clot retraction) (fig. 7 - 10 & ppt. 12)
Bio 307/607 lec 9
3.
- p. 6 -
Control of Hemostasis:
• anticoagulant processes: flowing blood washes away many uninvolved
clotting factors, diluting them and sending them to the liver for inactivation
(hemodynamic control)
- healthy endothelium releases several substances that inhibit platelet
aggregation (& degranulation), inhibit both extrinsic and intrinsic pathways (fig. 7 11 & ppt. 13); endothelial secretions notably include heparin that activates an
antithrombin present in plasma
• fibrinolysis: degrades clot, preparing way for healing
- mainly involves plasmin formed from inactive plasma protein,
plasminogen (fig. 7 - 12 & ppt. 15); various checks and balances in the system
promote enough coagulation to stem the blood loss (fig. 7 - 13 & ppt. 16)
4.
Bleeding Disorders:
• terms for blood loss: petechiae (minute pinpoint losses), purpura (somewhat
larger, more widespread patches of blood loss), ecchymoses (also known as bruises), &
hematoma (large mass of entrapped blood loss)
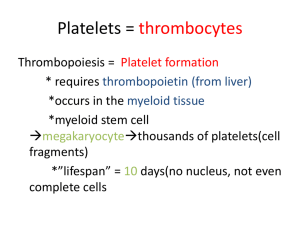
• thrombocytopenia: platelet deficiency resulting from impaired marrow
function (e.g. tumors, antitumor therapy) or excessive rate of platelet clearance from
blood (DIC, hemangiomas, immune destruction of platelets, & hypersplenism) (fig. 7
- 15 & ppt. 17)
• clotting factor disorders: may be genetically determined, or acquired
- genetic deficiencies include vWF (von Willebrand's disease), factors IX
and/or VIII (hemophilias)
- acquired deficiencies (fig. 7 - 16) are largely determined by some
impairment of liver function or inadequate supply of vitamin K; excessive clotting
in the body (disseminated intravascular coagulopathy - DIC) is also a known cause
Bio 307/607 lec 9
- p. 7 -
• anticoagulant therapy: overuse of heparin or warfarin (coumadin) may
cause dangerous internal bleeding, making it necessary to monitor such treatments
closely