01. atommolec.doc
advertisement

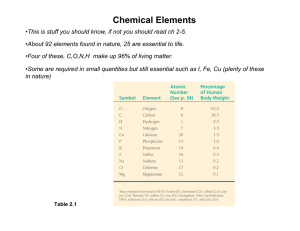
D’YOUVILLE COLLEGE BIOLOGY 102 - INTRODUCTORY BIOLOGY II LECTURE # 1 SIMPLE CHEMISTRY 1. Characteristics of Living Things: (chapter 1) • hierarchy of structure: high level of order of living things characterized by layers of increasing complexity; the smallest component layer that is capable of independent life is the cell (unit of life), which is itself composed of smaller layers (fig. 1 – 4 & ppt. 1) - layers include: atoms, ions & molecules, organelles, cells, tissues, organs, organ systems & the organism - each layer consists of lower layers & is incorporated into higher layers; a given layer exhibits properties that exceed the properties of its constituents (emergent properties); these derive from orderliness and interactions involving the constituents (fig. 2. – 3 & ppt. 2) • properties of living things (fig. 1 – 3): physicochemical systems with high level of order, capable of growth, differentiation & reproduction; capable of acquiring energy from environment to maintain order (fig. 1 – 5); capable of sensing and responding to environment; possession of many regulatory capabilities (fig. 1 – 13) (homeostasis) and adaptation to changing environmental circumstances 2. Atoms (fig. 2 – 5 & ppt. 3): • elementary particles: protons (positively charged), neutrons (neutral) & electrons (negatively charged); protons and neutrons have a mass approx. = 1; electrons have virtually no mass • nucleus of an atom contains the protons and (usually) neutrons • elements: materials made up exclusively of same type of atoms (table 2 – 1 & ppt. 4) Biology 102 - Spring ‘13 page 2 • atomic number: # of protons in nucleus; defines the element (fig. 2 – 9 & ppt. 5) • mass number: # of protons and neutrons Biology 102 - Spring ‘13 page 3 - isotopes – atoms of same atomic # but different mass #, e.g., 12C & 14C - unstable isotopes – isotopes that undergo nuclear decay over time through loss of elementary particles + energy; these are radioactive – useful in scientific studies (fig. 2 – 6) • orbitals (figs. 2 – 8, 2 – 10, ppts. 6 & 7): discrete energy levels that are occupied by electrons; orbitals make up shells with a characteristic maximum # of electrons: K = 2; L = 8; M = 8 (fig. 2 – 9 & ppt. 5) • stable configuration: maximal # of electrons in outer shell; virtually all of chemical reactions involve behavior of atoms seeking to achieve this stability 3. Ions & Molecules: • valence: # of electrons to lose (positive valences), gain (negative valences), or share to satisfy stable configuration (formation of bonds) • molecules: electron pairs shared (covalent bonds – figs. 2 – 11, 2 – 12 & ppt. 8); double bonds (two electron pairs shared) and triple bonds also exist - compounds – atoms combine to form compounds by covalent bonding; orbitals confer specific shapes (important for biological functions) upon molecules (fig. 2 – 17, ppts. 9 & 10) – particularly important for organic chemicals (fig. 2 – 18 & ppt. 11) Biology 102 - Spring ‘13 page 4 - polar molecules – molecules with bonds involving unequal sharing of electron pair; confers positive and negative ‘poles’ upon molecule (fig. 2 – 13 & ppt. 12) - hydrogen bonds – weak bonds between positive regions of one molecule and negative regions of another involving hydrogen atom of one molecule attracted, (but not lost) to another (figs. 2 – 16, 3 – 2 & ppts. 13 & 14) • ions: positive ions (= cations) have lost one or more electrons; negative ions (= anions) have gained one or more electrons - attraction of cations to anions constitutes ionic bond (figs. 2 – 3, 2 – 14, 2 - 15 & ppts. 15, 16 & 17)