Comparing I 2 Population Means (WIP)
advertisement
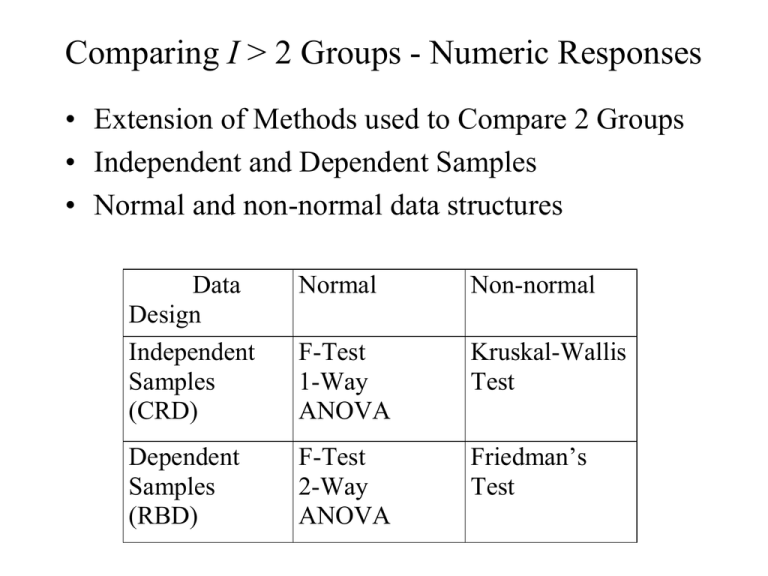
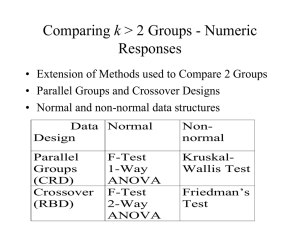
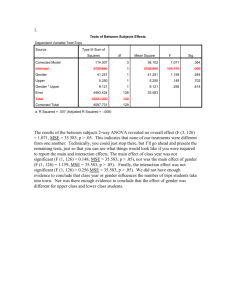
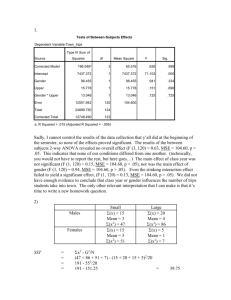
Comparing I > 2 Groups - Numeric Responses • Extension of Methods used to Compare 2 Groups • Independent and Dependent Samples • Normal and non-normal data structures Data Design Independent Samples (CRD) Normal Non-normal F-Test 1-Way ANOVA Kruskal-Wallis Test Dependent Samples (RBD) F-Test 2-Way ANOVA Friedman’s Test Independent Samples - Completely Randomized Design (CRD) • Controlled Experiments - Subjects assigned at random to one of the I treatments to be compared • Observational Studies - Subjects are sampled from I existing groups • Statistical model xij is a subject from group i: xij i ij where i is the population mean of group/treatment i , ij is a random error 1-Way ANOVA for Normal Data (CRD) • For each group obtain the mean, standard deviation, and sample size: xi xij j ni si 2 ( x x ) i ij j ni 1 • Obtain the overall mean and sample size N n1 nI n1 x1 nI x I i j xij x N N Analysis of Variance - Sums of Squares/Degrees of Freedom • Total Variation SST i 1 j 1 ( xij x) 2 I ni DFT N 1 • Among Group Variation SSG i 1 j 1 ( x i x) i 1 ni ( x i x) 2 I ni I 2 DFG I 1 • Within Group Variation SSE i 1 ji1 ( xij x i ) 2 i 1 (ni 1) si2 I n SST SSG SSE I DFT DFG DFE DFE N I Analysis of Variance Table and F-Test Source of Variation Treatments Error Total Sum of Squares SSG SSE SST Degrres of Freedom I-1 N-I N-1 Mean Square MSG=SSG/(I-1) MSE=SSE/(N-I) F F=MSG/MSE • H0: No differences among Group Means (1I) • HA: Group means are not all equal (Not all i are equal) MSG T .S . : Fobs MSE R.R. : Fobs F , I 1, N I P value : P( F Fobs ) (Table E ) Example - Relaxation Music in PatientControlled Sedation in Colonoscopy • Three Conditions (Treatments): – Music and Self-sedation (i = 1) – Self-Sedation Only (i = 2) – Music alone (i = 3) • Outcomes – Patient satisfaction score (all 3 conditions) – Amount of self-controlled dose (conditions 1 and 2) Source: Lee, et al (2002) Example - Relaxation Music in PatientControlled Sedation in Colonoscopy • Summary Statistics and Sums of Squares Calculations: Trt (i) 1 2 3 Total ni 55 55 55 165 Mean 7.8 6.8 7.4 overall mean=7.33 Std. Dev. 2.1 2.3 2.3 --- SSG 55(7.8 7.33) 2 55(6.8 7.33) 2 55(7.4 7.33) 2 31.29 DFG 3 1 2 SSE (55 1)( 2.1) 2 (55 1)( 2.3) 2 (55 1)( 2.3) 2 809.46 SST 31.29 809.46 840.75 DFE 165 3 162 DFT 2 162 164 Example - Relaxation Music in PatientControlled Sedation in Colonoscopy • Analysis of Variance and F-Test for Treatment effects Source of Variation Treatments Error Total Sum of Squares 31.29 809.46 840.75 Degrres of Freedom 2 162 164 Mean Square 15.65 5.00 •H0: No differences among Group Means (123) • HA: Group means are not all equal (Not all i are equal) 15.65 T .S . : Fobs 3.13 5.00 R.R. : Fobs F.05, 2 ,162 3.055 (Table E ) P value : P ( F 3.13) 0.05 F 3.13 Post-hoc Comparisons of Treatments • If differences in group means are determined from the F-test, researchers want to compare pairs of groups. Three popular methods include: – Dunnett’s Method - Compare active treatments with a control group. Consists of I-1 comparisons, and utilizes a special table. – Bonferroni’s Method - Adjusts individual comparison error rates so that all conclusions will be correct at desired confidence/significance level. Any number of comparisons can be made. – Tukey’s Method - Specifically compares all I(I-1)/2 pairs of groups. Utilizes a special table. Bonferroni’s Method (Most General) • Wish to make C comparisons of pairs of groups with simultaneous confidence intervals or 2-sided tests • Want the overall confidence level for all intervals to be “correct” to be 95% or the overall type I error rate for all tests to be 0.05 • For confidence intervals, construct (1-(0.05/C))100% CIs for the difference in each pair of group means (wider than 95% CIs) • Conduct each test at =0.05/C significance level (rejection region cut-offs more extreme than when =0.05) Bonferroni’s Method (Most General) • Simultaneous CI’s for pairs of group means: x x t i j / 2c, N I 1 1 MSE n n j i • If entire interval is positive, conclude i > j • If entire interval is negative, conclude i < j • If interval contains 0, cannot conclude i j Example - Relaxation Music in PatientControlled Sedation in Colonoscopy • C=3 comparisons: 1 vs 2, 1 vs 3, 2 vs 3. Want all intervals to contain true difference with 95% confidence • Will construct (1-(0.05/3))100% = 98.33% CIs for differences among pairs of group means t.05 / 2 ( 3),162 z.0083 2.40 MSE 5.00 n1 n2 n3 55 1 1 1 1 t.05 / 2 ( 3),162 MSE 2.40 5.00 1.02 n n 55 55 j i 1vs2 : (7.8 6.8) 1.02 (0.02,2.02) 1vs3 : (7.8 7.4) 1.02 (0.62,1.42) 2vs3 : (6.8 7.4) 1.02 (1.62,0.42) Note all intervals contain 0, but first is very close to 0 at lower end CRD with Non-Normal Data Kruskal-Wallis Test • Extension of Wilcoxon Rank-Sum Test to I>2 Groups • Procedure: – Rank the observations across groups from smallest (1) to largest (N = n1+...+nI), adjusting for ties – Compute the rank sums for each group: R1,...,RI . Note that R1+...+RI = N(N+1)/2 Kruskal-Wallis Test • H0: The I population distributions have same distribution • HA: Not all I distributions are identical 2 12 I Ri i 1 3( N 1) T .S . : H N ( N 1) ni 2 R.R. : H , I 1 P value : P( H ) 2 Post-hoc comparisons of pairs of groups can be made by pairwise application of rank-sum test with Bonferroni adjustment Example - Thalidomide for Weight Gain in HIV-1+ Patients with and without TB • I=4 Groups, n1=n2=n3=n4=8 patients per group (N=32) • Group 1: TB+ patients assigned Thalidomide • Group 2: TB- patients assigned Thalidomide • Group 3: TB+ patients assigned Placebo • Group 4: TB- patients assigned Placebo • Response - 21 day weight gains (kg) -- Negative values are weight losses Source: Klausner, et al (1996) Example - Thalidomide for Weight Gain in HIV-1+ Patients with and without TB TB+/Thal 9.0 (32) 6.0 (31) 4.5 (30) 2.0 (20.5) 2.5 (23) 3.0 (25) 1.0 (15.5) 1.5 (18.5) R1=195.5 TB-/Thal 2.5 (23) 3.5 (26.5) 4.0 (28.5) 1.0 (15.5) 0.5 (12) 4.0 (28.5) 1.5 (18.5) 2.0 (20.5) R2=173.0 TB+/Plac 0.0 (9) 1.0 (15.5) -1.0 (6) -2.0 (4) -3.0 (1.5) -3.0 (1.5) 0.5 (12) -2.5 (3) R3=52.5 TB-/Plac -0.5 (7) 0.0 (9) 2.5 (23) 0.5 (12) -1.5 (5) 0.0 (9) 1.0 (15.5) 3.5 (26.5) R4=107.0 12 (195.5) 2 (173.0) 2 (52.5) 2 (107.0) 2 3(33) 17.98 T .S . : H 32(33) 8 8 8 8 R.R. : H .205, 41 7.815 Weight Gain Example - SPSS Output F-Test and Post-Hoc Comparisons O W m d F S i a g f B 8 3 3 6 0 W 3 8 3 T 0 1 4 3 2 Mean of WTGAIN 1 0 -1 -2 TB+/Thalidom ide GROUP TB-/Thalidom ide TB+/Placebo TB-/Placebo Weight Gain Example - SPSS Output F-Test and Post-Hoc Comparisons C o m D ep M ean f i de n er en per d. ( er S I ( ( I J i J E ) g B ) ) B G T T T uk B B 89 1 6 18 7 3 4 1 6 T B 52 3 6 00 5 8* 4 4 1 T B 58 0 6 18 16 0* 4 4 T T B B 27 1 6 18 9 3 4 6 1 T B 20 2 6 03 4 5* 4 1 9 T B 27 8 6 02 96 8 4 1 T T B B 35 3 6 00 2 8 4 1 4 * T B 04 2 6 03 0 5 4 9 1 * T B 646 3 6 95 2 8 4 1 T T B B 416 0 6 18 8 0 4 4 * T B 896 8 6 02 7 8 4 1 T B 52 3 6 95 46 8 4 1 B T T B on B 99 1 6 0 7 3 4 0 4 9 T B 62 3 6 00 5 8* 4 1 4 T B 68 0 6 22 13 0* 4 7 T T B B 37 1 6 0 9 3 4 0 9 4 T B 31 2 6 04 38 5* 4 2 T B 37 8 6 12 99 8 4 4 T T B B 25 3 6 00 2 8 4 4 1 * T B 938 2 6 04 1 5 4 2 * T B 749 3 6 01 2 8 4 4 T T B B 313 0 6 22 8 0 4 7 * T B 999 8 6 12 7 8 4 4 T B 62 3 6 01 49 8 4 4 * . T he Weight Gain Example - SPSS Output Kruskal-Wallis H-Test n n N G W T 8 4 T 8 3 T 8 6 T 8 8 T 2 t a a G C d A a K b G Dependent Samples: Randomized Block Design (RBD) • I > 2 Treatments (groups) to be compared • J individuals receive each treatment (preferably in random order). Subjects are called Blocks. • Outcome when Treatment i is assigned to Subject j is labeled xij • Effect of Trt i is labeled i • Effect of Subject j is labeled bj • Random error term is labeled ij Dependent Samples - RBD • Model: xij i b j ij i b j ij • Test for differences among treatment effects: • H0: 1 ... I 0 (1 ... I) • HA: Not all i = 0 (Not all i are equal) RBD - ANOVA F-Test (Normal Data) • Data Structure: (I Treatments, J Subjects or Blocks) • Mean for Treatment i: x i. • Mean for Subject (Block) j: • Overall Mean: x. j x • Overall sample size: N = IJ • ANOVA:Treatment, Block, and Error Sums of Squares SST i 1 j 1 xij x I J SSB I x x SSG J i 1 x i . x I J j 1 .j 2 DFT IJ 1 2 DFG I 1 2 DFB J 1 SSE SST SSG SSB DFE ( I 1)( J 1) RBD - ANOVA F-Test (Normal Data) • ANOVA Table: Source Treatments Blocks Error Total SS SSG SSB SSE SST df I-1 J-1 (I-1)(J-1) IJ-1 MS MSG = SSG/(I-1) MSB = SSB/(J-1) MSE = SSE/[(I-1)(J-1)] •H0: 1 ... I 0 (1 ... I) • HA: Not all i = 0 T .S . : Fobs R.R. : Fobs (Not all i are equal) MSG MSE F , I 1,( I 1)( J 1) P value : P ( F Fobs ) F F = MSG/MSE Example - Theophylline Interaction • Goal: Determine whether Cimetidine or Famotidine interact with Theophylline • 3 Treatments: Theo/Cim, Theo/Fam, Theo/Placebo • 14 Blocks: Each subject received each treatment • Response: Theophylline clearance (liters/hour) Subject 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Source: Bachmann, et al (1995) TRT Mean T/C 3.69 3.61 1.15 4.02 1.00 1.75 1.45 2.59 1.57 2.34 1.31 2.43 2.33 2.34 2.26 T/F 5.13 7.04 1.46 4.44 1.15 2.11 2.12 3.25 2.11 5.20 1.98 2.38 3.53 2.33 3.16 T/P 5.88 5.89 1.46 4.05 1.09 2.59 1.69 3.16 2.06 4.59 2.08 2.61 3.42 2.54 3.08 BLK Mean 4.90 5.51 1.36 4.17 1.08 2.15 1.75 3.00 1.91 4.04 1.79 2.47 3.09 2.40 2.83 Example - Theophylline Interaction n - D I I I S q d S F S u i f g a C 7 5 4 8 0 I n 3 1 3 9 0 T 5 2 3 1 0 S 1 3 4 3 0 E 9 6 1 T 9 2 C 5 1 a R • The test for differences in mean theophylline clearance is given in the third line of the table •T.S.: Fobs=10.59 • R.R.: Fobs F.05,2,26 = 3.37 (From F-table) • P-value: .000 (Sig. Level) C Example - Theophylline Interaction Post-hoc Comparisons Bonferroni : x i. x j . t / 2 c ,( I 1)( J 1) Tukey : x i . x j . q.05, I ,( I 1)( J 1) 2 MSE J 1 MSE J o t 2.57 q 3.514 m D e M e a d e e r e e e S . I ( r r ( i I E J J g ) B B ) T T T u h h 3 0 3 3 6 6 1 7 5 * T h 3 0 3 3 6 6 2 7 5 * T T h h 3 0 3 3 6 6 1 5 7 * T h 3 2 0 0 0 6 8 1 1 T T h h 3 0 3 3 6 6 2 5 7 * T h 3 2 0 0 0 6 8 1 1 B T T o h h 3 0 9 7 6 6 1 8 4 * T h 3 0 9 7 6 6 2 8 4 * T T h h 3 0 7 9 6 6 1 4 8 * T h 3 0 6 6 0 6 0 2 2 T T h h 3 0 7 9 6 6 2 4 8 * T h 3 0 6 6 0 6 0 2 2 B a * . T h Example - Theophylline Interaction Plot of Data (Marginal means are raw data) Estimated Marginal Means of CLRNCE 7 6 5 TRT 4 Theophylline/Cimetid 3 ine 2 Theophylline/Famotid 1 ine 0 Theophylline/Placebo 1 3 2 5 4 SUBJECT 7 6 9 8 11 10 13 12 14 RBD -- Non-Normal Data Friedman’s Test • When data are non-normal, test is based on ranks • Procedure to obtain test statistic: – Rank the I treatments within each block (1=smallest, I=largest) adjusting for ties – Compute rank sums for treatments (Ri) across blocks – H0: The I populations are identical (1=...=I) – HA: Differences exist among the I group means 12 I 2 T .S . : Fr R 3J ( I 1) i 1 i IJ ( I 1) R.R. : Fr 2 , I 1 P value : P( 2 Fr ) Example - tmax for 3 formulation/fasting states • I=3 Treatments of Valproate: Capsule/Fasting (i=1), Capsule/nonfasting (i=2), Enteric-Coated/fasting (i=3) • J=11 subjects • Response - Time to maximum concentration (tmax) Subject Source: Carrigan, et al (1990) 1 2 3 4 5 6 7 8 9 10 11 Rank sum C/F 3.5 (2) 4.0 (2) 3.5 (2) 3.0 (1.5) 3.5 (1.5) 3.0 (1) 4.0 (2.5) 3.5 (2) 3.5 (1.5) 3.0 (1) 4.5 (2) T1=19.0 C/NF 4.5 (3) 4.5 (3) 4.5 (3) 4.5 (3) 5.0 (3) 5.5 (3) 4.0 (2.5) 4.5 (3) 5.0 (3) 4.5 (3) 6.0 (3) T2=32.5 EC/F 2.5 (1) 3.0 (1) 3.0 (1) 3.0 (1.5) 3.5 (1.5) 3.5 (2) 2.5 (1) 3.0 (1) 3.5 (1.5) 3.5 (2) 3.0 (1) T3=14.5 Example - tmax for 3 formulation/fasting states H0: The I populations are identical (1=...=I) HA: Differences exist among the I group means 12 T .S . : Fr 19.0 2 32.52 14.52 3(11)(3 1) 15.95 11(3)(3 1) R.R. : Fr .205,31 5.99 Data Sources • Lee,D.W., K.W. Chan, C.M. Poon, et al (2002). “Relaxation Music Decreases the Dose of Patient-Controlled Sedation During Colonoscopy: A Prospective Randomized Controlled Trial,” Gastrointestinal Endoscopy, 55:33-36. • Klausner,J.D., S. Makonkawkeyoon, P. Akarasewi, et al (1996). “The Effect of Thalidomide on the Pathogenesis of HIV-1 and M. tuberculosis Infection,” Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 11:247-257 • Bachmann, K., T.J. Sullivan, J.H. Reese, et al (1995). “Controlled Study of the Putative Interaction Between Famotidine and Theophylline in Patients with Chronic Obstructive Pulmonary Disorder,” Journal of Clinical Pharmacology, 35:529-535.