Form 6 Submission form
advertisement
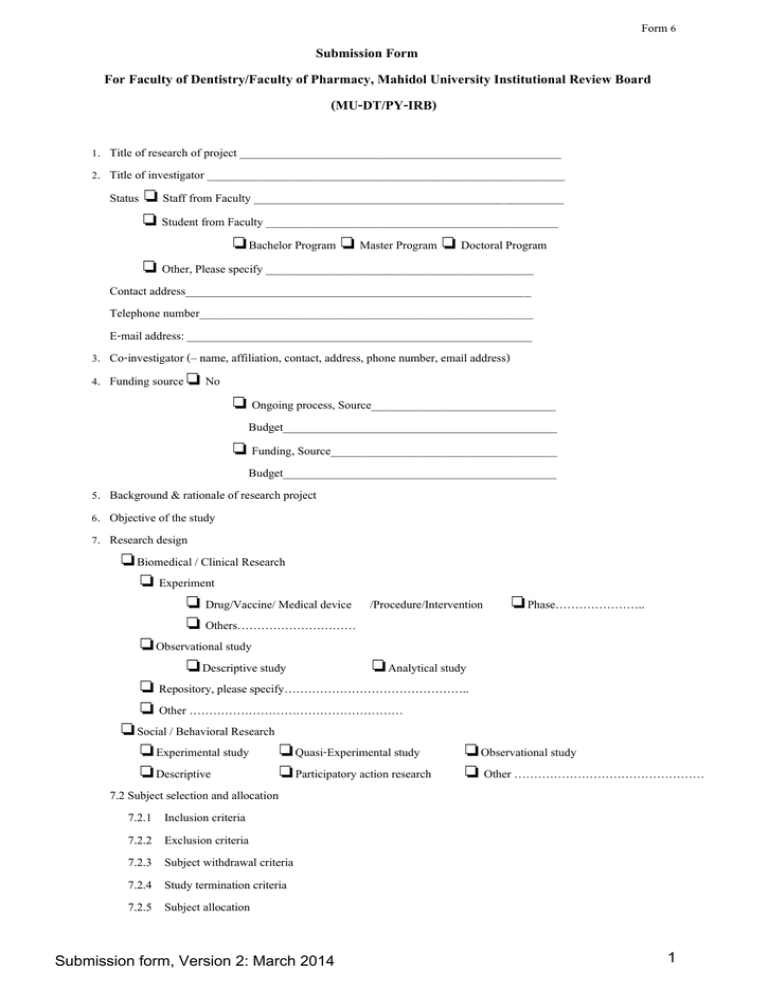
Form 6 Submission Form For Faculty of Dentistry/Faculty of Pharmacy, Mahidol University Institutional Review Board (MU-DT/PY-IRB) 1. Title of research of project ______________________________________________________ 2. Title of investigator ____________________________________________________________ Status ❏ Staff from Faculty ____________________________________________________ ❏ Student from Faculty _________________________________________________ ❏Bachelor Program ❏ Master Program ❏ Doctoral Program ❏ Other, Please specify _____________________________________________ Contact address__________________________________________________________ Telephone number________________________________________________________ E-mail address: __________________________________________________________ 3. Co-investigator (– name, affiliation, contact, address, phone number, email address) 4. Funding source ❏ No ❏ Ongoing process, Source_______________________________ Budget______________________________________________ ❏ Funding, Source______________________________________ Budget______________________________________________ 5. Background & rationale of research project 6. Objective of the study 7. Research design ❏Biomedical / Clinical Research ❏ Experiment ❏ Drug/Vaccine/ Medical device /Procedure/Intervention ❏Phase………………….. ❏ Others………………………… ❏Observational study ❏Descriptive study ❏Analytical study ❏ Repository, please specify……………………………………….. ❏ Other ……………………………………………… ❏Social / Behavioral Research ❏Experimental study ❏Quasi-Experimental study ❏Observational study ❏Descriptive ❏Participatory action research ❏ Other ………………………………………… 7.2 Subject selection and allocation 7.2.1 Inclusion criteria 7.2.2 Exclusion criteria 7.2.3 Subject withdrawal criteria 7.2.4 Study termination criteria 7.2.5 Subject allocation Submission form, Version 2: March 2014 1 Form 6 7.3 Sample size calculation 7.4 Sample size _________ cases ❏ Vulnerable subjects ❏ Children ❏ Mentally disable ❏ Chronic illness ❏ Others (please specify) _________________ ❏ Healthy volunteers ❏ Not vulnerable subjects 7.5 Replacement procedure (if subject withdraw from the study) 8. Study procedures 9. Study site ❏ Single center _____________________________________________________________ ❏ Multicenter ❏ within Thailand, please specify ___________________________ ❏ specify other countries and sites) __________________________ 10. Having specimen sending out of Mahidol University ❏ No ❏ Yes ( MTA will be required. Contact Center for Intellectual Property Management, Office of the President, Mahidol University 999 Phuttamonthon 4 Road, Salaya, Nakhon Pathom 73170 Thailand Tel. 02-849-6055-9) 11. Duration of study _______________________________________________________________________ 12. Data collection process (Case record form, questionnaire, interview, in-depth interview, other tools) 13. Outcome measurement/Data analysis - Primary outcome and secondary outcome (if any) - Assessment of efficacy - Assessment of safety - Statistical analysis or data analysis 14. Recruitment process (describe all process from the beginning of how you approach the volunteer, such as when, by whom. etc.) 15. Informed consent process (describe the process to get the Informed consent) which: ❏ 15.1 Immediately after recruitment process ❏ 15.2 Not immediately after recruitment process. Please specify when ____________by whom____ ❏ 15.3 Provide separate documents of volunteer information sheet and informed consent form ❏ 15.4 Waiver document for informed consent and provide only volunteer participant information (Must explain the reason of waiver) 16. Ethical consideration 16.1 Reason to be carried out in human, please specify the extent of problem that lead to research question, previous information and its controversy 16.2 Possible benefit for research subject personally and for all society 16.3 Foreseeable risk of research related injury 16.3.1 Explain information from previous study about severity and probability of adverse events 16.3.2 Management and Protection procedure for adverse events 16.3.3 Responsible person for research related injury 16.3.4 Contact person in case of adverse events Submission form, Version 2: March 2014 2 Form 6 16.3.5 For clinical research, how can principal investigator give the information about research participation to subject’s family doctor? 16.4 Reference for safety 16.5 Privacy and confidentiality protection, please specify the person who can reach the data; place to collect the data, archiving are living duration, and method of destroy the data. ❏ Coded data/specimen ❏ Recorded by ❏ photograph ❏ VDO ❏ tape recorder ❏ None of the above 17. Submitted documents 1. ❏ MU-DT/PY-IRB Submission form: 1 original, 3 copies with electronic file 2. ❏ Protocol/Proposal: 1 original, 3 copies with electronic file 3. ❏ Volunteers Information Sheet: 20 copies with electronic file 4. ❏ Informed Consent Form: 20 copies with electronic file 5. ❏ Principal Investigator’s Curriculum Vitae: 4 copies 6. ❏ Commitment for Research Conduct: 1 original, 3 copies 7. ❏ The research tools used for collecting data; i.e. questionnaires, interview/observation guide: 4 copies with electronic file 8. ❏ Case record form/case report forms: 4 copies with electronic file 9. ❏ Advertisement: 4 copies 10. ❏ Documents to be given to volunteers (specify type) (if any 4 copies) 11. ❏ In case of drug trial. Specify the registration number by the Food and Drug Administration Ministry of Public Health or document to bring drug research and other necessary documents relating to the drug (Drug Registered Number, IND, Investigator Brochure, Other Forms or Reports required by the MU-DT/PY-IRB) 2 copies 12. ❏ Permission from authorized to use medical records (In case of retrospective medical record review):4 copies 13. ❏ Permission from authorized person to use stored specimen (In case of stored specimen):4 copies 14. ❏ Permission from authorized to collect data and/or use research area: 4 copies 15. ❏ Permission from authorized person to exempt medical/dental fee: 4 copies 16. For post graduate students, attach the following additional document 16.1 ❏ The Faculty of Graduate Studies' Administrative Order for the thesis topic and the appointment of the Thesis Advisory Committee or Form GR.39 if the thesis proposal examination is ongoing: 4 copies each 16.2 ❏ Advisor’s and student’s curriculum vitae: 4 copies each 16.3 ❏ The certificate of attendance or the certificate of achievement from the conference on Ethics in Human Research: 4 copies 17. ❏ Certificate of Approval (Being subproject of the large project that has been approved by the Ethics Committee) 4 copies 18. ❏ Certificate of Approval (The project has been approved by the Ethics Committee of the data collecting site) 4 copies 19. ❏ Proof of fee payment or document for fee exemption Submission form, Version 2: March 2014 3 Form 6 18. Commitments 1. I, as the principal investigator, and my co-investigators as listed and signed in this documents will conduct this study according to the protocol approved by MU-DT/PY-IRB. I will conduct the informed consent process by providing adequate information as approved and sufficient opportunity to consider whether or not to participate to potential subjects, with respect for person, without coercion and undue influence. 2. I will obtain pre-approval of any changes in research activity and informed research subjects about the change for their considerations to continuing their participations in the study. 3. I will report MU-DT/PY-IRB all adverse events and unanticipated events and will do my best to help research subjects. 4. I will provide reports concerning the progress of the research annually or when requested. 5. I and my co-investigator have adequate knowledge and training in procedural intervention needed in conducting research and providing care for any research-related injury to research subjects. Signature of principal investigator_____________________, date___________ (…………...(Name)………………) Signature of co-investigator__________________________, date___________ (……………..….(Name)……………..….) 19. Permission from Thesis Advisor/ Direct Superior Authorized to Approve Research Projects Signature ______________________, date_________ (……..……..(Name)………….…..) Thesis Advisor/ Direct Superior Authorized to Approve Research Projects Remarks:1. Since this MU-DT/PY-IRB submission form is applied to all types of research, if any statement is not applicable to your research, put “NA” (not applicable) to that statement. (Do not skip any statement or leave it blank) 2. If you have any question, please contact: Office of MU-DT/PY-IRB, Faculty of Dentistry, Mahidol University The 50th Anniversary of HRH Princess Mahachakri Sirindhorn Building, 11st Floor, 6 Yothi Street, Rajthevi, Bangkok 10400, THAILAND Tel/Fax: (662)-200-7622 Email: dtpyirb@mahidol.ac.th Submission form, Version 2: March 2014 4




